Impact of prenatal immune challenge on the demyelination injury during adulthood
Affiliations
Affiliations
- Faculty of Medicine, Department of Physiology, Kuwait University, Safat, Kuwait.
Abstract
Aim: Brain inflammation is associated with several brain diseases such as multiple sclerosis (MS), a disease characterized by demyelination. Whether prenatal immune challenge affects demyelination-induced inflammation in the white matter during adulthood is unclear. In the present study, we used a well-established experimental model of focal demyelination to assess whether prenatal immune challenge affects demyelination-induced inflammation.
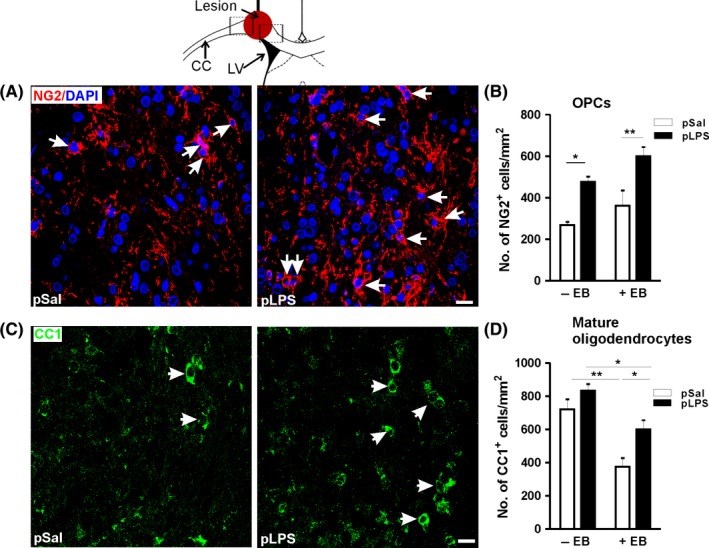
Methods: Pregnant rats were injected with either lipopolysaccharide (100 μg/kg, ip) or pyrogen-free saline. A 2 μL solution of the gliotoxin ethidium bromide (0.04%) was stereotaxically infused into the corpus callosum of adult male offspring. The extent of demyelination lesion was assessed using Luxol fast blue (LFB) staining. Oligodendrocyte precursor cells, mature oligodendrocytes, markers of cellular gliosis, and inflammation were monitored in the vicinity of the demyelination lesion area.
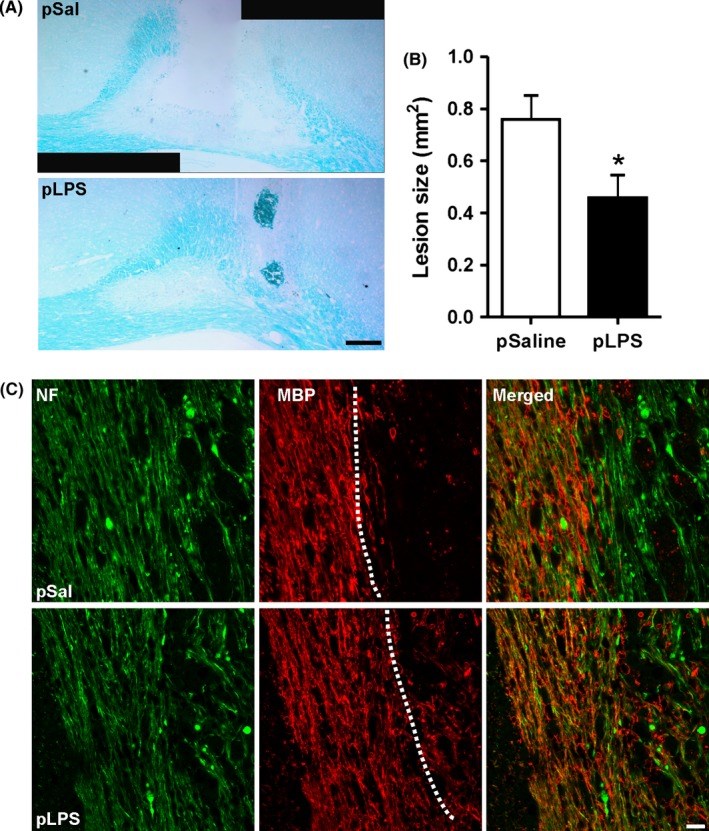
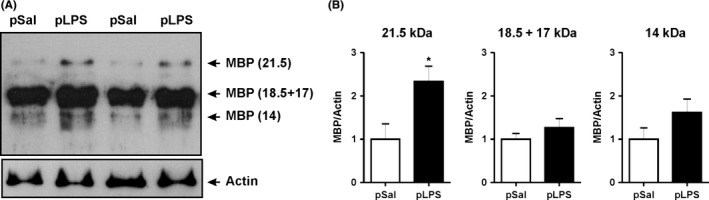
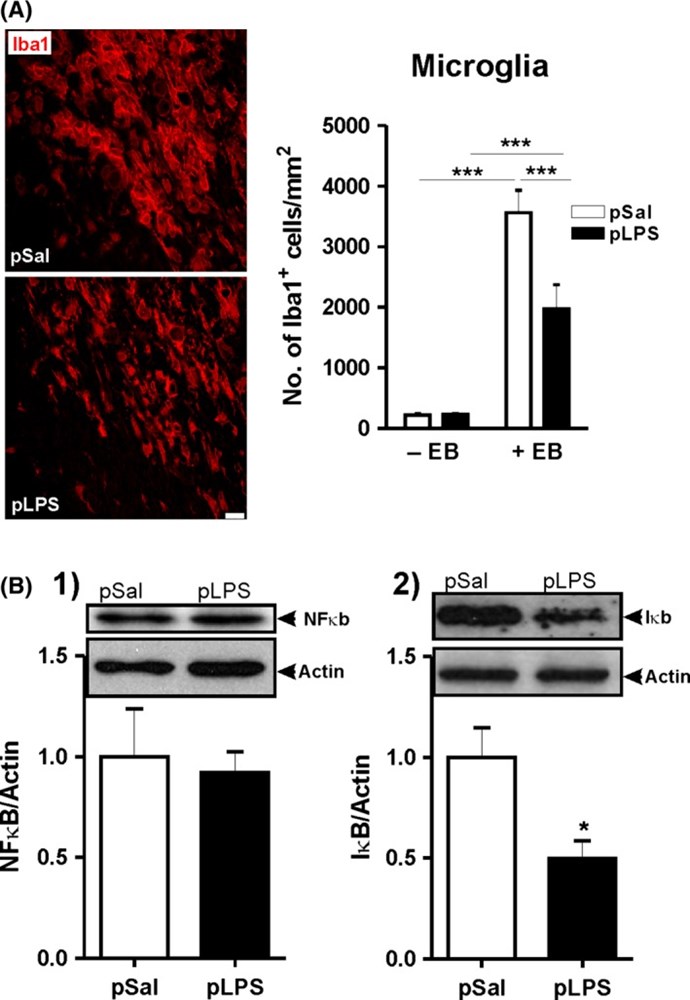
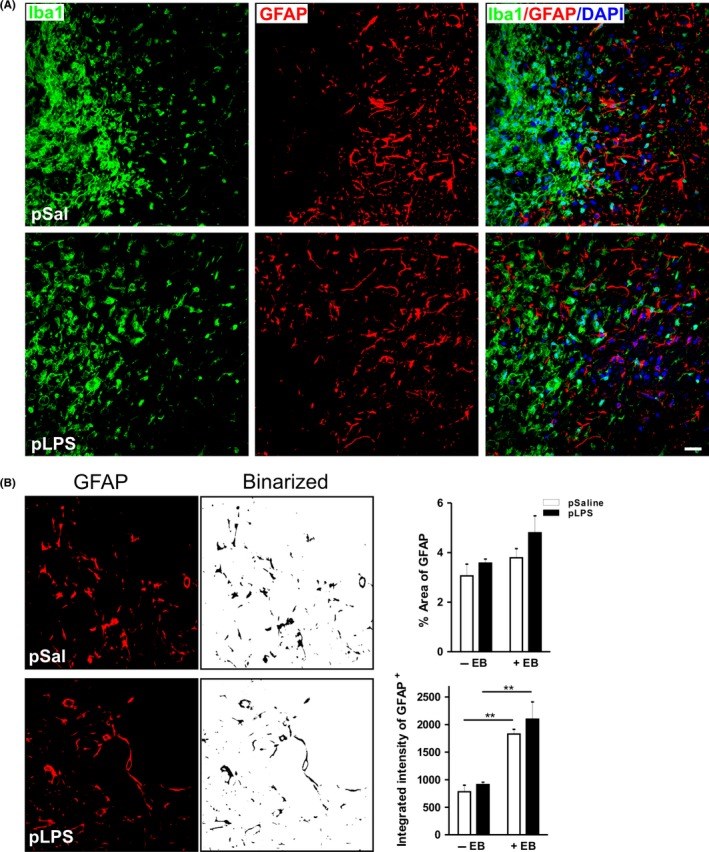
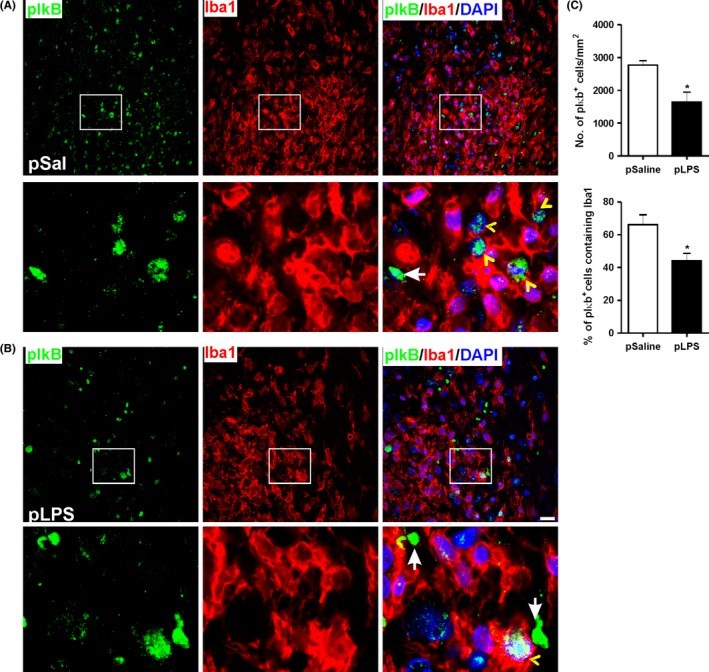
Results: Prenatal lipopolysaccharide reduced the size of the demyelination lesion during adulthood. This reduced lesion was associated with enhanced density of mature oligodendrocytes and reduced density of microglial cells in the vicinity of the demyelination lesion. Such reduction in microglial cell density was accompanied by a reduced activation of the nuclear factor κB signaling pathway.
Conclusion: These data strongly suggest that prenatal immune challenge dampens the extent of demyelination during adulthood likely by reprogramming the local brain inflammatory response to demyelinating insults.
Keywords: maternal inflammation; microglia; myelin basic protein; oligodendrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
Kalakh S, Mouihate A.Neuropathol Appl Neurobiol. 2015 Dec;41(7):964-82. doi: 10.1111/nan.12237. Epub 2015 May 30.PMID: 25786683
Demyelination-Induced Inflammation Attracts Newly Born Neurons to the White Matter.
Kalakh S, Mouihate A.Mol Neurobiol. 2017 Oct;54(8):5905-5918. doi: 10.1007/s12035-016-0127-5. Epub 2016 Sep 23.PMID: 27660277
Naeimi R, Safarpour F, Hashemian M, Tashakorian H, Ahmadian SR, Ashrafpour M, Ghasemi-Kasman M.Neurosci Lett. 2018 May 1;674:1-10. doi: 10.1016/j.neulet.2018.03.018. Epub 2018 Mar 9.PMID: 29530814
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.
Keirstead HS, Blakemore WF.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15.PMID: 10635029 Review.
Prenatal inflammation exposure-programmed cardiovascular diseases and potential prevention.
Deng Y, Song L, Nie X, Shou W, Li X.Pharmacol Ther. 2018 Oct;190:159-172. doi: 10.1016/j.pharmthera.2018.05.009. Epub 2018 May 25.PMID: 29803628 Review.
Cited by
Xu MY, Wang YF, Wei PJ, Gao YQ, Zhang WT.CNS Neurosci Ther. 2019 Jun;25(6):734-747. doi: 10.1111/cns.13102. Epub 2019 Jan 28.PMID: 30689302 Free PMC article.