Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation
Affiliations
Affiliations
- Division of Immunology, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass; Division of Pediatric Immunology and Allergy, Meram Medical Faculty, Necmettin Erbakan University, Konya, Turkey.
- Division of Immunology, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass.
- Program in Molecular and Cellular Medicine, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass.
- Division of Pediatric Immunology and Allergy, Meram Medical Faculty, Necmettin Erbakan University, Konya, Turkey.
- Division of Pediatric Immunology, Behcet Uz State Hospital, Izmir, Turkey.
- Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait.
- Department of Pathology, Boston Children's Hospital, Harvard Medical School, Boston, Mass.
- Division of Pediatric Immunology, Ege University, Izmir, Turkey.
- Division of Immunology, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass; Program in Molecular and Cellular Medicine, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass.
- Division of Immunology, Department of Pediatrics, Boston Children's Hospital, Harvard Medical School, Boston, Mass. Electronic address: talal.chatila@childrens.harvard.edu.
Abstract
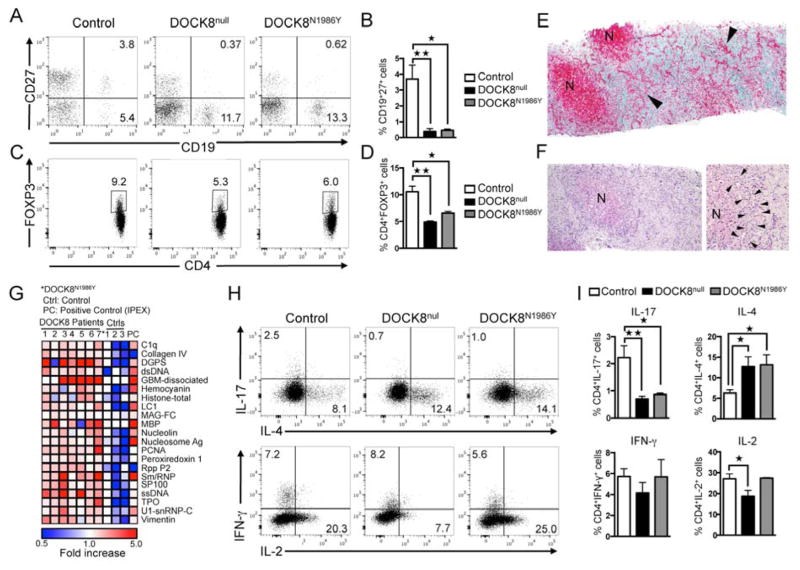
Background: The autosomal recessive hyper-IgE syndrome (HIES) caused by dedicator of cytokinesis 8 (DOCK8) deficiency shares clinical features with autosomal dominant HIES because of signal transducer and activator of transcription 3 (STAT3) mutations, including recurrent infections and mucocutaneous candidiasis, which are suggestive of TH17 cell dysfunction. The mechanisms underlying this phenotypic overlap are unclear.
Objective: We sought to elucidate common mechanisms operating in the different forms of HIES.
Methods: We analyzed the differentiation of CD4+ TH cell subsets in control and DOCK8-deficient subjects. We also examined the role of DOCK8 in regulating STAT3 activation in T cells. TH cell differentiation was analyzed by ELISA, flow cytometry, and real-time PCR measurements of cytokines and TH cell transcription factors. The interaction of DOCK8 and STAT3 signaling pathways was examined by using flow cytometry, immunofluorescence, coimmunoprecipitation, and gene expression analysis.
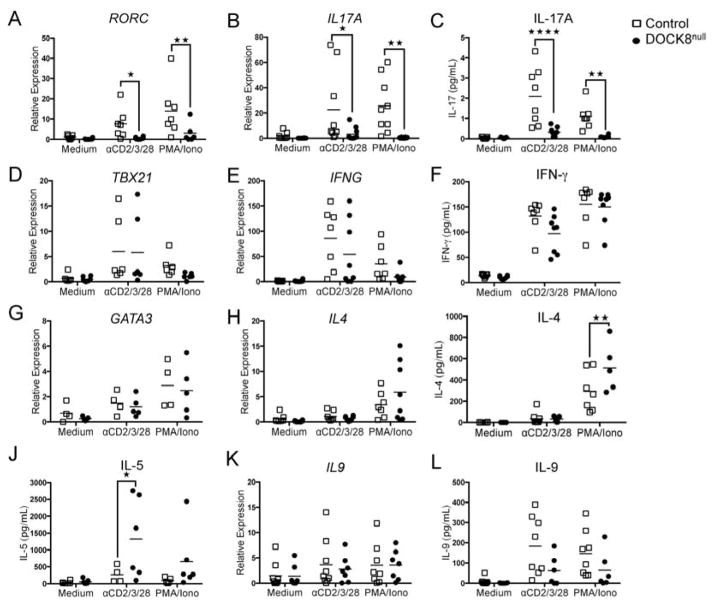
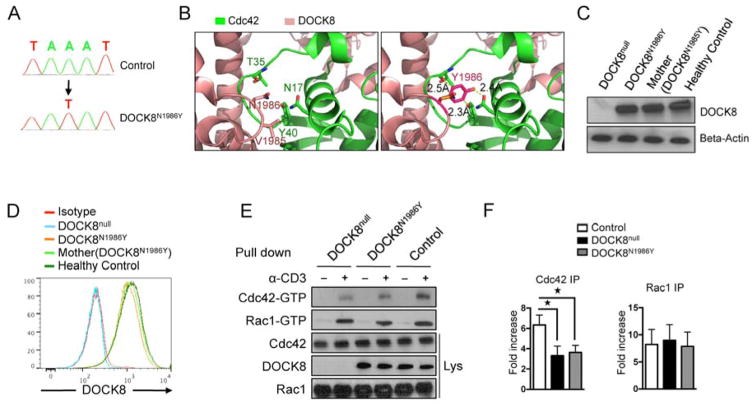
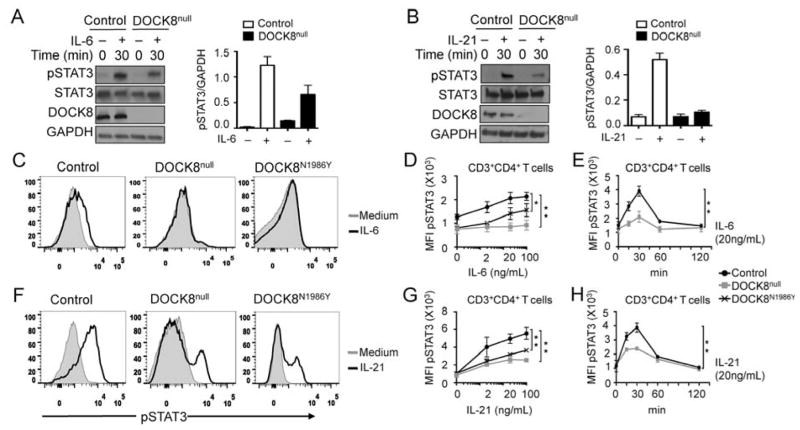
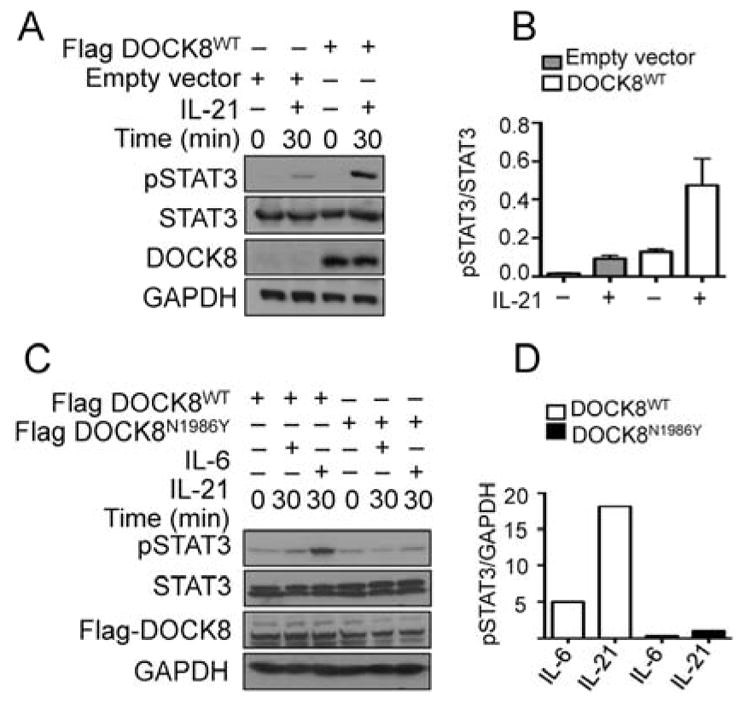
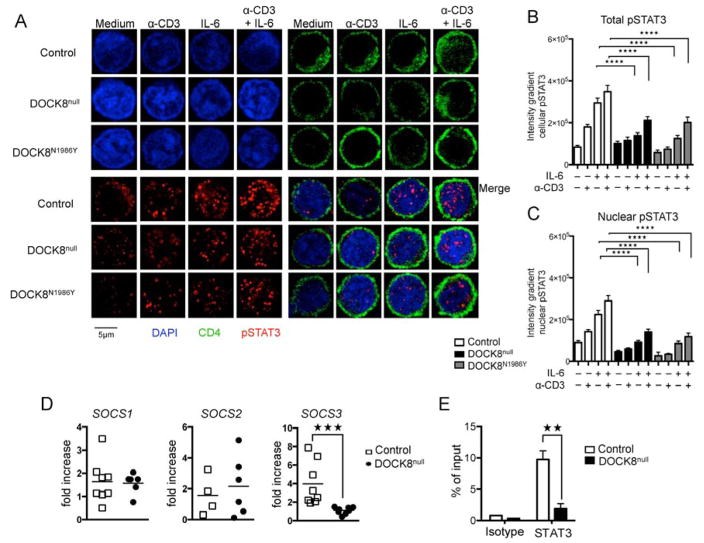
Results: There was a profound block in the differentiation of DOCK8-deficient naive CD4+ T cells into TH17 cells. A missense mutation that disrupts DOCK8 guanine nucleotide exchange factor (GEF) activity while sparing protein expression also impaired TH17 cell differentiation. DOCK8 constitutively associated with STAT3 independent of GEF activity, whereas it regulated STAT3 phosphorylation in a GEF activity-dependent manner. DOCK8 also promoted STAT3 translocation to the nucleus and induction of STAT3-dependent gene expression.
Conclusion: DOCK8 interacts with STAT3 and regulates its activation and the outcome of STAT3-dependent TH17 differentiation. These findings might explain the phenotypic overlap between DOCK8 deficiency and autosomal dominant HIES.
Keywords: Cell division cycle 42; T(H)17; dedicator of cytokinesis 8; guanine nucleotide exchange factor; hyper-IgE syndrome; mucocutaneous candidiasis; signal transducer and activator of transcription 3; suppressor of cytokine signaling 3.
Figures
Similar articles
The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency.
Engelhardt KR, Gertz ME, Keles S, Schäffer AA, Sigmund EC, Glocker C, Saghafi S, Pourpak Z, Ceja R, Sassi A, Graham LE, Massaad MJ, Mellouli F, Ben-Mustapha I, Khemiri M, Kilic SS, Etzioni A, Freeman AF, Thiel J, Schulze I, Al-Herz W, Metin A, Sanal Ö, Tezcan I, Yeganeh M, Niehues T, Dueckers G, Weinspach S, Patiroglu T, Unal E, Dasouki M, Yilmaz M, Genel F, Aytekin C, Kutukculer N, Somer A, Kilic M, Reisli I, Camcioglu Y, Gennery AR, Cant AJ, Jones A, Gaspar BH, Arkwright PD, Pietrogrande MC, Baz Z, Al-Tamemi S, Lougaris V, Lefranc G, Megarbane A, Boutros J, Galal N, Bejaoui M, Barbouche MR, Geha RS, Chatila TA, Grimbacher B.J Allergy Clin Immunol. 2015 Aug;136(2):402-12. doi: 10.1016/j.jaci.2014.12.1945. Epub 2015 Feb 25.PMID: 25724123 Free PMC article.
Tangye SG, Pillay B, Randall KL, Avery DT, Phan TG, Gray P, Ziegler JB, Smart JM, Peake J, Arkwright PD, Hambleton S, Orange J, Goodnow CC, Uzel G, Casanova JL, Lugo Reyes SO, Freeman AF, Su HC, Ma CS.J Allergy Clin Immunol. 2017 Mar;139(3):933-949. doi: 10.1016/j.jaci.2016.07.016. Epub 2016 Aug 20.PMID: 27554822
Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern.
Boos AC, Hagl B, Schlesinger A, Halm BE, Ballenberger N, Pinarci M, Heinz V, Kreilinger D, Spielberger BD, Schimke-Marques LF, Sawalle-Belohradsky J, Belohradsky BH, Przybilla B, Schaub B, Wollenberg A, Renner ED.Allergy. 2014 Jul;69(7):943-53. doi: 10.1111/all.12416.PMID: 24898675
DOCK8 deficiency: Insights into pathophysiology, clinical features and management.
Biggs CM, Keles S, Chatila TA.Clin Immunol. 2017 Aug;181:75-82. doi: 10.1016/j.clim.2017.06.003. Epub 2017 Jun 15.PMID: 28625885 Free PMC article. Review.
Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care.
Farmand S, Sundin M.Curr Opin Hematol. 2015 Jan;22(1):12-22. doi: 10.1097/MOH.0000000000000104.PMID: 25469836 Review.
Cited by
Zhou X, Hu J, Xu D, Zhang S, Wang Q.Exp Ther Med. 2023 Feb 10;25(3):134. doi: 10.3892/etm.2023.11833. eCollection 2023 Mar.PMID: 36845964 Free PMC article.
Diagnostic challenge in a series of eleven patients with hyper IgE syndromes.
Yaakoubi R, Mekki N, Ben-Mustapha I, Ben-Khemis L, Bouaziz A, Ben Fraj I, Ammar J, Hamzaoui A, Turki H, Boussofara L, Denguezli M, Haddad S, Ouederni M, Bejaoui M, Chan KW, Lau YL, Mellouli F, Barbouche MR, Ben-Ali M.Front Immunol. 2023 Jan 10;13:1057679. doi: 10.3389/fimmu.2022.1057679. eCollection 2022.PMID: 36703986 Free PMC article.
The ups and downs of STAT3 function: too much, too little and human immune dysregulation.
Mackie J, Ma CS, Tangye SG, Guerin A.Clin Exp Immunol. 2023 Apr 25;212(2):107-116. doi: 10.1093/cei/uxad007.PMID: 36652220
Insights into the pathogenesis of allergic disease from dedicator of cytokinesis 8 deficiency.
Su HC.Curr Opin Immunol. 2023 Feb;80:102277. doi: 10.1016/j.coi.2022.102277. Epub 2022 Dec 9.PMID: 36508760 Review.
Zhang L, Cao Y, Dai X, Zhang X.Front Pharmacol. 2022 Nov 1;13:1065029. doi: 10.3389/fphar.2022.1065029. eCollection 2022.PMID: 36386145 Free PMC article. Review.