Characterization of Patients in the International Severe Asthma Registry with High Steroid Exposure Who Did or Did Not Initiate Biologic Therapy
Wenjia Chen 1, Mohsen Sadatsafavi 2, Trung N Tran 3, Ruth B Murray 4, Chong Boon Nigel Wong 1, Nasloon Ali 4 5, Cono Ariti 4 5, Esther Garcia Gil 6, Anthony Newell 5 7, Marianna Alacqua 8, Mona Al-Ahmad 9, Alan Altraja 10, Riyad Al-Lehebi 11 12, Mohit Bhutani 13, Leif Bjermer 14, Anne Sofie Bjerrum 15, Arnaud Bourdin 16, Lakmini Bulathsinhala 4 5, Anna von Bülow 17, John Busby 18, Giorgio Walter Canonica 19 20, Victoria Carter 4 5, George C Christoff 21, Borja G Cosio 22, Richard W Costello 23, J Mark FitzGerald 24, João A Fonseca 25, Kwang Ha Yoo 26, Liam G Heaney 27, Enrico Heffler 19 20, Mark Hew 28 29, Ole Hilberg 30, Flavia Hoyte 31 32, Takashi Iwanaga 33, David J Jackson 34 35, Rupert C Jones 36, Mariko Siyue Koh 37 38, Piotr Kuna 39, Désirée Larenas-Linnemann 40, Sverre Lehmann 41, Lauri A Lehtimäki 42 43, Juntao Lyu 5 7, Bassam Mahboub 44 45, Jorge Maspero 46 47, Andrew N Menzies-Gow 48, Concetta Sirena 49, Nikolaos Papadopoulos 50 51, Andriana I Papaioannou 52, Luis Pérez de Llano 53 54, Diahn-Warng Perng 55 56, Matthew Peters 57, Paul E Pfeffer 58 59, Celeste M Porsbjerg 17, Todor A Popov 60, Chin Kook Rhee 61, Sundeep Salvi 62, Camille Taillé 63, Christian Taube 64, Carlos A Torres-Duque 65, Charlotte S Ulrik 66, Seung Won Ra 67, Eileen Wang 31 32, Michael E Wechsler 68, David B Price 4 5 69
Affiliations
Affiliations
- Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore.
- Respiratory Evaluation Sciences Program, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, Canada.
- AstraZeneca, Gaithersburg, MD, USA.
- Optimum Patient Care, Cambridge, UK.
- Observational and Pragmatic Research Institute, Singapore, Singapore.
- AstraZeneca, Barcelona, Spain.
- Optimum Patient Care, Queensland, VIC, Australia.
- AstraZeneca, Cambridge, UK.
- Microbiology Department, Faculty of Medicine, Kuwait University, Al-Rashed Allergy Center, Ministry of Health, Kuwait City, Kuwait.
- Department of Pulmonology, University of Tartu and Lung Clinic, Tartu University Hospital, Tartu, Estonia.
- Department of Pulmonology, King Fahad Medical City, Riyadh, Saudi Arabia.
- College of Medicine, Alfaisal University, Riyadh, Saudi Arabia.
- Department of Medicine, Division of Pulmonary Medicine, University of Alberta, Western Canada, AB, Canada.
- Department of Clinical Sciences, Respiratory Medicine and Allergology, Skåne University Hospital, Lund University, Lund, Sweden.
- Department of Respiratory Medicine and Allergy, Aarhus University Hospital, Jutland, Aarhus, Denmark.
- PhyMedExp, Univ Montpellier, CNRS, INSERM, CHU Montpellier, Montpellier, France.
- Respiratory Research Unit, Bispebjerg University Hospital, Copenhagen, Denmark.
- Centre for Public Health, Queen's University Belfast, Belfast, Northern Ireland.
- Personalized Medicine, Asthma and Allergy, Humanitas Clinical and Research Center IRCCS, Milan, Italy.
- Department of Biomedical Sciences, Humanitas University, Milan, Italy.
- Medical University-Sofia, Faculty of Public Health, Sofia, Bulgaria.
- Son Espases University Hospital-IdISBa-Ciberes, Mallorca, Spain.
- Department of Respiratory Medicine, Clinical Research Centre, Smurfit Building Beaumont Hospital, RCSI, Dublin, Ireland.
- Department of Medicine, the University of British Columbia, Vancouver, BC, Canada.
- Comunity Health, Information and Decision Sciences Department (MEDCIDS) & Center for Health Technology and Services Research (CINTESIS), Faculty of Medicine of University of Porto, Porto, Portugal.
- KonKuk University School of Medicine in Seoul, Seoul, Korea.
- Wellcome-Wolfson Centre for Experimental Medicine, Queen's University Belfast, Belfast, Northern Ireland.
- Allergy, Asthma & Clinical Immunology Service, Alfred Health, Melbourne, VIC, Australia.
- Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
- Medical Department, Vejle University Hospital, Jutland, Vejle, Denmark.
- Department of Medicine, Division of Allergy and Clinical Immunology, National Jewish Health, Denver, CO, USA.
- Department of Internal Medicine, Division of Allergy & Clinical Immunology, University of Colorado School of Medicine, Aurora, CO, USA.
- Center for General Medical Education and Clinical Training, Kindai University Hospital, Osakasayama, Japan.
- UK Severe Asthma Network and National Registry, Guy's and St Thomas' NHS Trust, London, UK.
- School of Immunology & Microbial Sciences, King's College London, London, UK.
- Research and Knowledge Exchange, Plymouth Marjon University, Plymouth, UK.
- Respiratory & Critical Care Medicine, Singapore General Hospital, Singapore, Singapore.
- SingHealth Duke-NUS Lung Centre, Singapore, Singapore.
- Division of Internal Medicine, Asthma and Allergy Medical University of Łódź, Łódź, Poland.
- Directora Centro de Excelencia en Asma y Alergia, Hospital Médica Sur, Ciudad de México, Mexico.
- Section of Thoracic Medicine, Department of Clinical Science, University of Bergen, Bergen, Norway.
- Allergy Centre, Tampere University Hospital, Tampere, Finland.
- Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
- College of Medicine, University of Sharjah, Sharjah, United Arab Emirates.
- Rashid Hospital, Dubai Health Authority, Dubai, United Arab Emirates.
- Clinical Research for Allergy and Respiratory Medicine, CIDEA Foundation, Buenos Aires, Argentina.
- University Career of Specialists in Allergy and Clinical Immunology at the Buenos Aires University School of Medicine, Buenos Aires, Argentina.
- Royal Brompton & Harefield Hospitals, London, UK.
- Severe Asthma Network in Italy (SANI), Milano, Italy.
- Division of Infection, Immunity & Respiratory Medicine, University of Manchester, Manchester, UK.
- Allergy Department, 2nd Pediatric Clinic, University of Athens, Athens, Greece.
- 2nd Respiratory Medicine Department, National and Kapodistrian University of Athens Medical School, Attikon University Hospital, Athens, Greece.
- Pneumology Service, Lucus Augusti University Hospital, EOXI Lugo, Lugo, Spain.
- Biodiscovery Research Group, Health Research Institute of Santiago de Compostela, Santiago de Compostela, Spain.
- Division of Clinical Respiratory Physiology Chest Department, Taipei Veterans General Hospital, Taipei, Taiwan.
- COPD Assembly of the Asian Pacific Society of Respirology Hongo, Bunkyo-ku, Tokyo, Japan.
- Department of Thoracic Medicine, Concord Hospital, Sydney, NSW, Australia.
- Department of Respiratory Medicine, Barts Health NHS Trust, London, UK.
- Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
- University Hospital "sv. Ivan Rilski", Sofia, Bulgaria.
- Department of Internal Medicine, Division of Pulmonary and Critical Care Medicine, Seoul St. Mary's Hospital, College of Medicine, the Catholic University of Korea, Seoul, South Korea.
- Pulmocare Research and Education Foundation, Pune, India.
- Department of Respiratory Diseases, Bichat Hospital, AP-HP Nord-Université de Paris, Paris, France.
- Department of Pulmonary Medicine, University Medical Center Essen-Ruhrlandklinik, Essen, Germany.
- CINEUMO, Respiratory Research Center, Fundación Neumológica Colombiana, Bogotá, Colombia.
- Department of Respiratory Medicine, Copenhagen University Hospital-Hvidovre, Hvidovre, Denmark.
- Department of Internal Medicine, Division of Pulmonology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea.
- Department of Medicine, NJH Cohen Family Asthma Institute, National Jewish Health, Denver, CO, USA.
- Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK.
Abstract
Background: Many severe asthma patients with high oral corticosteroid exposure (HOCS) often do not initiate biologics despite being eligible. This study aimed to compare the characteristics of severe asthma patients with HOCS who did and did not initiate biologics.
Methods: Baseline characteristics of patients with HOCS (long-term maintenance OCS therapy for at least 1 year, or ≥4 courses of steroid bursts in a year) from the International Severe Asthma Registry (ISAR; https://isaregistries.org/), who initiated or did not initiate biologics (anti-lgE, anti-IL5/5R or anti-IL4R), were described at the time of biologic initiation or registry enrolment. Statistical relationships were tested using Pearson's chi-squared tests for categorical variables, and t-tests for continuous variables, adjusting for potential errors in multiple comparisons.
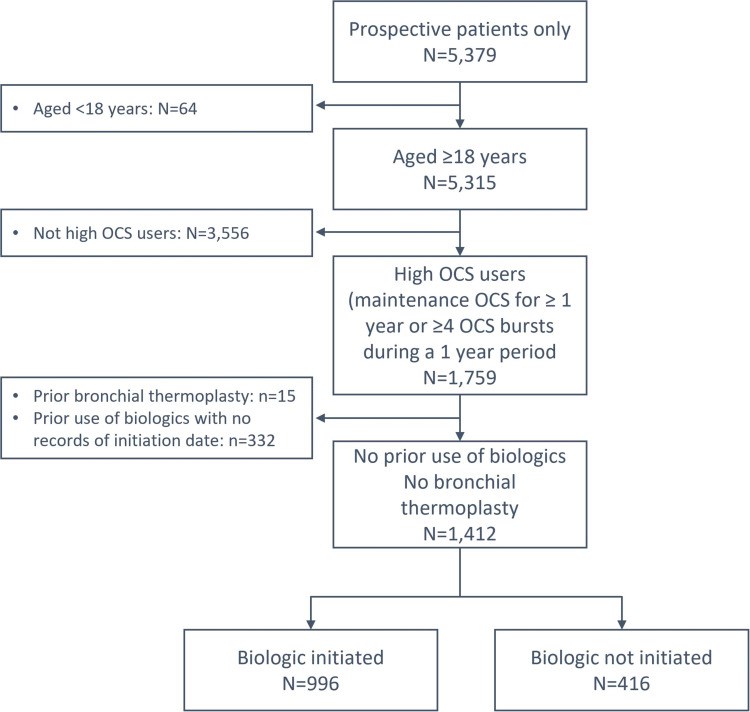
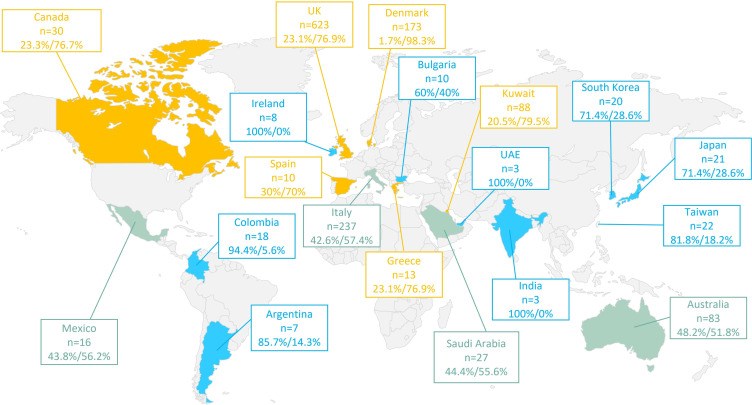
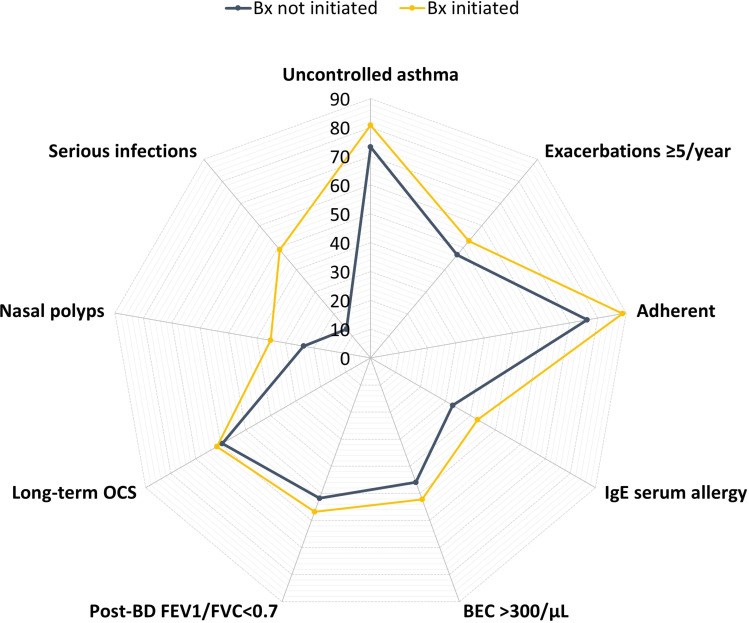
Results: Between January 2015 and February 2021, we identified 1412 adult patients with severe asthma from 19 countries that met our inclusion criteria of HOCS, of whom 996 (70.5%) initiated a biologic and 416 (29.5%) did not. The frequency of biologic initiation varied across geographical regions. Those who initiated a biologic were more likely to have higher blood eosinophil count (483 vs 399 cells/µL, p=0.003), serious infections (49.0% vs 13.3%, p<0.001), nasal polyps (35.2% vs 23.6%, p<0.001), airflow limitation (56.8% vs 51.8%, p=0.013), and uncontrolled asthma (80.8% vs 73.2%, p=0.004) despite greater conventional treatment adherence than those who did not start a biologic. Both groups had similar annual asthma exacerbation rates in the previous 12 months (5.7 vs 5.3, p=0.147).
Conclusion: Around one third of severe HOCS asthma patients did not receive biologics despite a similar high burden of asthma exacerbations as those who initiated a biologic therapy. Other disease characteristics such as eosinophilic phenotype, serious infectious events, nasal polyps, airflow limitation and lack of asthma control appear to dictate biologic use.
Keywords: biologics; patient characteristics; real-world; severe asthma; treatment pattern.
Conflict of interest statement
Mohsen Sadatsafavi has received honoraria from AZ, BI, and GSK for purposes unrelated to the content of this manuscript and has received research funding from AZ and BI directly into his research account from AZ for unrelated projects. Trung N. Tran is an employee of AstraZeneca. AstraZeneca is a co-funder of the International Severe Asthma Registry. Nigel Chong Boon Wong reports grants from Optimum Patient Care Global, grants from AstraZeneca Ltd, during the conduct of the study. Nasloon Ali was an employee of Observational and Pragmatic Research Institute (OPRI) at the time this research was conducted. OPRI conducted this study in collaboration with Optimum Patient Care and AstraZeneca. Con Ariti is an employee of the Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care and AstraZeneca. Esther Garcia Gil was an employee of AstraZeneca at the time this research was conducted. AstraZeneca is a co-funder of the International Severe Asthma Registry. Anthony Newell was an employee of Optimum Patient Care (OPC) at the time this research was conducted. OPC is a co-funder of the International Severe Asthma Registry. Marianna Alacqua was an employee of AstraZeneca at the time this research was conducted. AstraZeneca is a co-funder of the International Severe Asthma Registry. Mona Al-Ahmad has received advisory board and speaker fees from AstraZeneca, Sanofi, Novartis, and GlaxoSmithKline. Alan Altraja has received lecture fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, Norameda, Novartis, Orion, Sanofi, and Zentiva; personal fees from Teva, Shire Pharmaceuticals and CSL Behring, sponsorships from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MSD, Norameda, Sanofi, and Novartis; and has been a member of advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi, and Teva. Riyad Al-Lehebi has given lectures at meetings supported by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Sanofi, reports personal fees from Novartis and participated in advisory board fees from GlaxoSmithKline. Mohit Bhutani: has received advisory board and speaker fees from AstraZeneca, GlaxoSmithKline, Pfizer, Sanofi Genzyme, Covis pharmaceuticals; has been an investigator on clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Sanofi Genzyme, Boehringer Ingelheim Leif Bjermer has (in the last three years) received lecture or advisory board fees from Alk-Abello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, Sanofi, Genzyme/Regeneron, and Teva. Anne-Sofie Bjerrum has received lecture fees from Astra Zeneca, GlaxoSmithKline, Novartis. Arnaud Bourdin has received industry-sponsored grants from AstraZeneca-MedImmune, Boehringer-Ingelheim, Cephalon/Teva, GlaxoSmithKline, Novartis, Sanofi-Regeneron and consultancies with AstraZeneca-MedImmune, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Regeneron-Sanofi, Med-in-Cell, Actelion, Merck, Roche, and Chiesi. Lakmini Bulathsinhala is an employee of the Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care and AstraZeneca. Anna von Bülow reports speakers fees and consultancy fees from AstraZeneca, GSK and Novartis, outside the submitted work. She has also attended advisory board for Novartis and AstraZeneca. John Busby reports personal fees from NuvoAir, outside the submitted work. Giorgio Walter Canonica has received research grants, as well as lecture or advisory board fees from A. Menarini, Alk-Albello, Allergy Therapeutics, Anallergo, AstraZeneca, MedImmune, Boehringer Ingelheim, Chiesi Farmaceutici, Circassia, Danone, Faes, Genentech, Guidotti Malesci, GlaxoSmithKline, Hal Allergy, Merck, MSD, Mundipharma, Novartis, Orion, Sanofi Aventis, Sanofi, Genzyme/Regeneron, Stallergenes, UCB Pharma, Uriach Pharma, Teva, Thermo Fisher, and Valeas. Victoria Carter is an employee of Optimum Patient Care, a co-funder of the International Severe Asthma Registry and reports grants from Optimum Patient Care Global, during the conduct of the study Borja G. Cosio declares grants from Chiesi, AstraZeneca, Menarini, Teva and GSK; personal fees for advisory board activities from Chiesi, GSK, Novartis, Sanofi, Menarini, Teva and AstraZeneca; and payment for lectures/speaking engagements from Chiesi, Novartis, GSK, Menarini, Sanofi, Teva and AstraZeneca, outside the submitted work. Richard W. Costello has received honoraria for lectures from Aerogen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Teva. He is a member of advisory boards for GlaxoSmithKline and Novartis, has received grant support from GlaxoSmithKline and Aerogen, has received personal fees from Respirasense, personal fees from PfizerBioNTech, during the conduct of the study and has patents in the use of acoustics in the diagnosis of lung disease, assessment of adherence and prediction of exacerbations, issued to 212. J. Mark FitzGerald reports grants from AstraZeneca, GSK, Sanofi Regeneron, Novartis paid directly to UBC. Personal fees for lectures and attending advisory boards: AstraZeneca, GSK, Sanofi Regeneron, TEVA. João A Fonseca reports grants from or research agreements with AstraZeneca, Mundipharma, Sanofi Regeneron and Novartis. Personal fees for lectures and attending advisory boards: AstraZeneca, GSK, Mundipharma, Novartis, Sanofi Regeneron and TEVA. Liam G. Heaney declares he has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Circassia, Evelo Biosciences, Hoffmann la Roche, GlaxoSmithKline, Novartis, Theravance and Teva; he has taken part in asthma clinical trials sponsored by Boehringer Ingelheim, Hoffmann la Roche, and GlaxoSmithKline for which his institution received remuneration; he is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche, and Janssen. Enrico Heffler participates in speaking activities and industry advisory committees for AstraZeneca, Sanofi-Genzyme, GSK, Novartis, Regeneron, Stallergenes-Greer, TEVA, Circassia and Nestlè Purina, grants from AstraZeneca and GSK Mark Hew declares grants and other advisory board fees (made to his institutional employer) from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, and Seqirus, for unrelated projects. Flavia Hoyte declares honoraria from AstraZeneca. She has been an investigator on clinical trials sponsored by GlaxoSmithKline, Genentech, Teva, Sanofi and National Institute of Allergy and Infectious Diseases (NIAID), for which her institution has received funding. Takashi Iwanaga declares grants from Astellas, Boehringer Ingelheim, Daiichi-Sankyo, Kyorin, MeijiSeika Pharma, Teijin Pharma, Ono, and Taiho, and lecture fees from Kyorin, GlaxoSmithKline, Sanofi and AstraZeneca. David J. Jackson has received advisory board and speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Teva, Napp, Chiesi, Sanofi Regeneron, Novartis and research grant funding from AstraZeneca. Rupert C. Jones declares grants from Astra Zeneca, Glaxo Smith Kline, Novartis OPRI and Teva and personal fees for consultancy, speakers’ fees or travel support from Astra Zeneca, Boehringer Ingelheim, Glaxo Smith Kline, Novartis and OPRI. Mariko Siyue Koh reports grant support from AstraZeneca, and honoraria for lectures and advisory board meetings paid to her hospital (Singapore General Hospital) from GlaxoSmithKline, AstraZeneca, Sanofi and Boehringer Ingelheim, outside the submitted work. Piotr Kuna reports personal fees from Adamed, personal fees from AstraZeneca, personal fees from Berlin Chemie Manarini, personal fees from Boehringer Ingelheim, personal fees from Lekam, personal fees from Novartis, personal fees from Chiesi, personal fees from Polpharma, personal fees from Sanofi, personal fees from Teva, personal fees from Zentiva, personal fees from FAES, personal fees from Glenmark, outside the submitted work Désirée Larenas Linnemann reports speaker or personal fees from ALK, Alakos, Armstrong, AstraZeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Gossamer, Grunenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Purina institute, Sanofi, Siegfried, UCB, Carnot, Viatris, and grants from Sanofi, AbbVie, ALK, AstraZeneca, Circassia, Chiesi, GSK, Lilly, Novartis, Pfizer, Purina Institute and UCB, outside the submitted work Sverre Lehmann declares receipt of lecture (personal) and advisory board (to employer) fees from AstraZeneca, Boehringer Ingelheim, and Novartis. Lauri A. Lehtimäki declares personal fees for consultancy, lectures and attending advisory boards from ALK, AstraZeneca, Boehringer Ingelheim, Circassia, Chiesi, GlaxoSmithKline, Mundipharma, Novartis, Orion Pharma, Sanofi, and Teva. Juntao Lyu is an employee of Optimum Patient Care (OPC). OPC is a co-funder of the International Severe Asthma Registry. Jorge Maspero reports grants and personal fees from AstraZeneca, Sanofi, Novartis, and GSK, personal fees from IMMUNOTEK, personal fees from SANOFI, outside the submitted work. Andrew N. Menzies-Gow has attended advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Regeneron, Sanofi and Teva, andhas received speaker fees from AstraZeneca, Novartis, Teva and Sanofi. He has participated in research with AstraZeneca for which his institution has been remunerated and has attended international conferences with Teva. He has had consultancy agreements with AstraZeneca and Sanofi. Nikolaos G. Papadopoulos declares research support from Gerolymatos, Menarini, Nutricia, and Vian; and consultancy/speaker fees from ASIT, AZ, Boehringer Ingelheim, GSK, HAL Allergy, Medscape, Menarini, MSD, Mylan, Novartis, and Nutricia, OM Pharma, Sanofi, and Takeda, grant from Capricare. Andriana I. Papaioannou has received fees and honoraria from Menarini, GSK, Novartis, Elpen, Boehringer Ingelheim, AstraZeneca, and Chiesi. Luis Perez-de-Llano declares non-financial support, personal fees, and grants from Teva, Sanofi and AstraZeneca, Chesi; non-financial support and personal fees from Boehringer Ingelheim, Esteve, GlaxoSmithKline, Mundipharma, and Novartis; personal fees from MSD, TECHDOW PHARMA, Leo-Pharma, GEBRO and GILEAD; non-financial support from Menairi, grants, non-financial support from FAES during the conduct of the study. Diahn-Warng Perng (Steve) received sponsorship to attend or speak at international meetings, honoraria for lecturing or attending advisory boards, and research grants from the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Daiichi Sankyo, Shionogi and Orient Pharma Matthew Peters declares personal fees and non-financial support from AstraZeneca and GlaxoSmithKline. Paul E. Pfeffer has attended advisory boards for AstraZeneca, Sanofi and GSK; has given lectures at meetings supported by AstraZeneca and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Sanofi and Novartis, for which his institution received remuneration; non-financial support from Chiesi; and has a current research grant funded by GlaxoSmithKline. Celeste M. Porsbjerg has attended advisory boards for AstraZeneca, Novartis, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, MSD, Sanofi-Genzyme, GlaxoSmithKline, and Novartis; and has received educational and research grants from AstraZeneca, Novartis, TEVA, GlaxoSmithKline, ALK, and Sanofi-Genzyme. Todor A. Popov declares relevant research support from Novartis and Chiesi Pharma. Chin Kook Rhee declares consultancy and lecture fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mundipharma, MSD, Novartis, Sandoz, Sanofi, Takeda, and Teva-Handok. Sundeep Salvi declares research support and speaker fees from Cipla, Glenmark, GSK Camille Taillé has received lecture or advisory board fees and grants to her institution from AstraZeneca, Sanofi, GlaxoSmithKline, Chiesi and Novartis, for unrelated projects. Carlos A. Torres-Duque has received fees as advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has taken part in clinical trials from AstraZeneca, Novartis and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundacion Neumologica Colombiana from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Grifols and Novartis. Charlotte S. Ulrik has attended advisory boards for AstraZeneca, ALK-Abello, GSK, Boehringer-Ingelheim, Novartis, Chiesi, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Sandoz, Mundipharma, Chiesi, Boehringer-Ingelheim, Orion Pharma, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, Merck, InsMed, ALK-Abello, Sanofi-Genzyme, GlaxoSmithKline, Boehringer-Ingelheim, Regeneron, Chiesi and Novartis; and has received educational and research grants from AstraZeneca, MundiPharma, Boehringer-Ingelheim, Novartis, TEVA, GlaxoSmithKline and Sanofi-Genzyme; has received personal fees from Pfizer. Seung Won Ra has received lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis. Eileen Wang has received honoraria from AstraZeneca, GlaxoSmithKline, Wefight, and Clinical Care Options. She has been an investigator on clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Genentech, Novartis, Teva, and Sanofi, for which her institution has received funding. She has received personal fees from Wefight. Michael E. Wechsler reports receiving consulting honoraria from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Novartis, Regeneron, Sanofi and Teva; personal fees from Amgen, Avalo, Cerecor, Cytoreason, Eli Lilly, Equillium, Incyte, Kinaset, Phylaxis, Pulmatrix, Rapt and Sound Biologics. David B. Price has advisory board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
Figures
Similar articles
Pfeffer PE, Ali N, Murray R, Ulrik C, Tran TN, Maspero J, Peters M, Christoff GC, Sadatsafavi M, Torres-Duque CA, Altraja A, Lehtimäki L, Papadopoulos NG, Salvi S, Costello RW, Cushen B, Heffler E, Iwanaga T, Al-Ahmad M, Larenas-Linnemann D, Kuna P, Fonseca JA, Al-Lehebi R, Rhee CK, Perez-de-Llano L, Perng Steve DW, Mahboub B, Wang E, Goh C, Lyu J, Newell A, Alacqua M, Belevskiy AS, Bhutani M, Bjermer L, Bjornsdottir U, Bourdin A, Bulow AV, Busby J, Canonica GW, Cosio BG, Dorscheid DR, Muñoz-Esquerre M, FitzGerald JM, Gil EG, Gibson PG, Heaney LG, Hew M, Hilberg O, Hoyte F, Jackson DJ, Koh MS, Ko HB, Lee JH, Lehmann S, Chaves Loureiro C, Lúðvíksdóttir D, Menzies-Gow AN, Mitchell P, Papaioannou AI, Popov TA, Porsbjerg CM, Salameh L, Sirena C, Taillé C, Taube C, Tohda Y, Wechsler ME, Price DB.Allergy. 2023 Mar 17. doi: 10.1111/all.15711. Online ahead of print.PMID: 36929509
Menzies-Gow AN, McBrien C, Unni B, Porsbjerg CM, Al-Ahmad M, Ambrose CS, Dahl Assing K, von Bülow A, Busby J, Cosio BG, FitzGerald JM, Garcia Gil E, Hansen S, aHeaney LG, Hew M, Jackson DJ, Kallieri M, Loukides S, Lugogo NL, Papaioannou AI, Larenas-Linnemann D, Moore WC, Perez-de-Llano LA, Rasmussen LM, Schmid JM, Siddiqui S, Alacqua M, Tran TN, Suppli Ulrik C, Upham JW, Wang E, Bulathsinhala L, Carter VA, Chaudhry I, Eleangovan N, Murray RB, Price CA, Price DB.J Asthma Allergy. 2022 Jan 13;15:63-78. doi: 10.2147/JAA.S328653. eCollection 2022.PMID: 35046670 Free PMC article.
Abbas F, Georas S, Cai X, Khurana S.Ann Allergy Asthma Immunol. 2021 Dec;127(6):655-660.e1. doi: 10.1016/j.anai.2021.08.416. Epub 2021 Sep 3.PMID: 34481992
Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma.
Katsaounou P, Buhl R, Brusselle G, Pfister P, Martínez R, Wahn U, Bousquet J.Respir Med. 2019 Apr;150:51-62. doi: 10.1016/j.rmed.2019.02.003. Epub 2019 Feb 7.PMID: 30961951 Review.
Biologics for oral corticosteroid-dependent asthma.
Yılmaz İ.Allergy Asthma Proc. 2020 May 1;41(3):151-157. doi: 10.2500/aap.2020.41.200015.PMID: 32375958 Review.
Cited by
Chen W, Reddel HK, FitzGerald JM, Beasley R, Janson C, Sadatsafavi M.Respir Res. 2023 May 2;24(1):120. doi: 10.1186/s12931-023-02409-2.PMID: 37131185 Free PMC article. Clinical Trial.
KMEL References
References
-
- Reddel HK, Taylor DR, Bateman ED., et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi:10.1164/rccm.200801-060ST - DOI - PubMed
-
- Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Org J. 2019;12:100007. doi:10.1016/j.waojou.2018.12.001 - DOI - PMC - PubMed
-
- Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi:10.1136/thoraxjnl-2015-207630 - DOI - PubMed
-
- Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75:1023–1042. doi:10.1111/all.14221 - DOI - PubMed
-
- ISAR Study Group. International Severe Asthma Registry (ISAR): mission Statement. Chest. 2020;157:805–814. - PubMed
-
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry (ISAR). Chest. 2020;157:805–814. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2018. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-rev.... Accessed September 28, 2022.
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Soc Series B. 1995;57:289–300.
-
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled Phase 3 trial. Lancet. 2016;388:2128–2141. doi:10.1016/S0140-6736(16) - DOI - PubMed
-
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi:10.1016/S2213-2600(15) - DOI - PubMed
-
- Tinoco EM, Gigante AR, Fernandes AL, et al. Impact of biologic therapy in severe asthma with nasal polyps. Eur Respir J. 2021:58. doi:10.1183/13993003.congress-2021.PA3739 - DOI
-
- Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. doi:10.1016/S0140-6736(19) - DOI - PubMed