SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry
Jean Liew # 1, Milena Gianfrancesco # 2, Carly Harrison 3, Zara Izadi 4, Stephanie Rush 5, Saskia Lawson-Tovey 6 7, Lindsay Jacobsohn 2, Clairissa Ja 8, Kimme L Hyrich 9, Laure Gossec 10 11, Anja Strangfeld 12, Loreto Carmona 13, Martin Schäfer 14, Elsa Frãzao-Mateus 15, Inita Bulina 16, Frances Stafford 17, Abdurrahman Tufan 18, Christine Graver 19, Gözde Kübra Yardımcı 20 21, Julija Zepa 16, Samar Al Emadi 22, Claire Cook 23, Fatemah Abutiban 24, Dfiza Dey 25 26, Genevieve Katigbak 27, Lauren Kaufman 28, Emily Kowalski 29, Marco Ulises Martínez-Martínez 30 31, Naomi J Patel 32, Greta Reyes-Cordero 33, Evelyn Salido 34, Ellison Smith 35 36, David Snow 37, Jeffrey Sparks 38, Leanna Wise 39, Suleman Bhana 40, Monique Gore-Massy 41, Rebecca Grainger 42 43, Jonathan Hausmann 44 45, Emily Sirotich 46, Paul Sufka 47, Zachary Wallace 32 48, Pedro M Machado 49 50, Philip C Robinson 51 52, Jinoos Yazdany 53
Affiliations
Affiliations
- Medicine, Section of Rheumatology, Boston University, Boston, Massachusetts, USA liew.jw@gmail.com.
- Department of Medicine, Division of Rheumatology, University of California, San Francisco, San Francisco, California, USA.
- LupusChat, New York, New York, USA.
- Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California, USA.
- Division of Rheumatology, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
- Centre for Genetics and Genomics Versus Arthritis, The University of Manchester, Manchester, UK.
- National Institute for Health Research Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester, UK.
- Division of Rheumatology, Department of Medicine, University of California, San Francisco, California, USA.
- University of Manchester, Manchester, UK.
- INSERM, Institut Pierre Louis d'Epidémiologie et de Santé Publique, INSERM, Sorbonne Universite, Paris, France.
- APHP, Rheumatology Department, Hopital Universitaire Pitie Salpetriere, Paris, France.
- Forschungsbereich Epidemiologie, Deutsches Rheuma-Forschungszentrum Berlin, Berlin, Germany.
- Instituto de Salud Musculoesquelética (INMUSC), Madrid, Spain.
- Epidemiology and Health Services Research, German Rheumatism Research Center Berlin, Berlin, Germany.
- Portuguese League Against Rheumatic Diseases (LPCDR), Lisbon, Portugal.
- Paul Stradins Clinical University Hospital, Riga, Latvia.
- Blackrock Clinic, Blackrock, Ireland.
- Gazi University, Ankara, Turkey.
- Royal Hampshire County Hospital, Winchester, UK.
- Department of Internal Medicine, Hacettepe University, Ankara, Turkey.
- Hacettepe University, Ankara, Turkey.
- Hamad Medical Corporation, Doha, Qatar.
- Rheumatology, Massachusetts General Hospital, Boston, Massachusetts, USA.
- Jaber Al Ahmed Al Jaber Al Sabah Hospital, Surra, Kuwait.
- University of Ghana Medical School, Accra, Ghana.
- Korle Bu Teaching Hospital, Accra, Ghana.
- Makati Medical Center, Makati City, Philippines.
- Rheumatology Associates Louisville, Louisville, Kentucky, USA.
- Inflammation and Immunity, Brigham and Women's Hospital, Boston, Massachusetts, USA.
- Rheumatology, Hospital Central "Dr Ignacio Morones Prieto", San Luis Potosí, Mexico.
- Faculty of Medicine, Universidad Autónoma de San Luis Potosí, San Luis, Mexico.
- Massachusetts General Hospital, Boston, Massachusetts, USA.
- Autonomous University of Chihuahua, Chihuahua, Mexico.
- University of the Philippines Manila, Manila, Philippines.
- University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
- Asheville Arthritis & Osteoporosis Center, Asheville, North Carolina, USA.
- Cape Fear Arthritis Care, Leland, North Carolina, USA.
- Department of Medicine, Division of Rheumatology, Immunology and Allergy, Brigham and Women's Hospital, Boston, Massachusetts, USA.
- Department of Internal Medicine, Division of Rheumatology, University of Southern California, Los Angeles, California, USA.
- Crystal Run Healthcare, Middletown, New York, USA.
- Lupus Foundation of America, Washington, DC, USA.
- Department of Medicine, University of Otago, Wellington, Wellington, New Zealand.
- University Of Otago, Wellington, New Zealand.
- Rheumatology, Boston Children's Hospital, Boston, Massachusetts, USA.
- Division of Rheumatology and Clinical Immunology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
- Medicine, McMaster University, Hamilton, Ontario, Canada.
- Healthpartners, St Paul, Minnesota, USA.
- Harvard Medical School, Boston, Massachusetts, USA.
- MRC Centre for Neuromuscular Diseases, University College London, London, UK.
- Rheumatology, University College London Centre for Rheumatology, London, UK.
- Faculty of Medicine, The University of Queensland, Herston, Queensland, Australia.
- Metro North Hospital and Health Service, Royal Brisbane and Women's Hospital Health Service District, Herston, Queensland, Australia.
- Medicine/Rheumatology, University of California, San Francisco, California, USA.
Abstract
Objective: While COVID-19 vaccination prevents severe infections, poor immunogenicity in immunocompromised people threatens vaccine effectiveness. We analysed the clinical characteristics of patients with rheumatic disease who developed breakthrough COVID-19 after vaccination against SARS-CoV-2.
Methods: We included people partially or fully vaccinated against SARS-CoV-2 who developed COVID-19 between 5 January and 30 September 2021 and were reported to the Global Rheumatology Alliance registry. Breakthrough infections were defined as occurring ≥14 days after completion of the vaccination series, specifically 14 days after the second dose in a two-dose series or 14 days after a single-dose vaccine. We analysed patients' demographic and clinical characteristics and COVID-19 symptoms and outcomes.
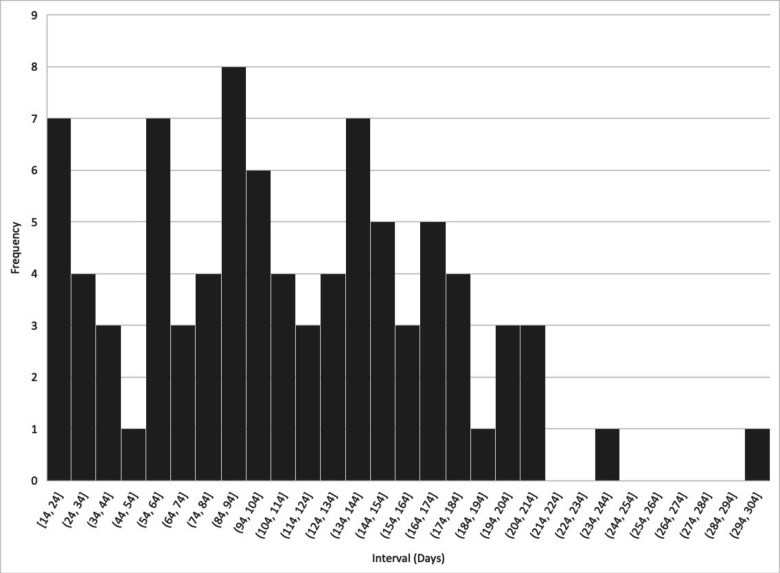
Results: SARS-CoV-2 infection was reported in 197 partially or fully vaccinated people with rheumatic disease (mean age 54 years, 77% female, 56% white). The majority (n=140/197, 71%) received messenger RNA vaccines. Among the fully vaccinated (n=87), infection occurred a mean of 112 (±60) days after the second vaccine dose. Among those fully vaccinated and hospitalised (n=22, age range 36-83 years), nine had used B cell-depleting therapy (BCDT), with six as monotherapy, at the time of vaccination. Three were on mycophenolate. The majority (n=14/22, 64%) were not taking systemic glucocorticoids. Eight patients had pre-existing lung disease and five patients died.
Conclusion: More than half of fully vaccinated individuals with breakthrough infections requiring hospitalisation were on BCDT or mycophenolate. Further risk mitigation strategies are likely needed to protect this selected high-risk population.
Keywords: COVID-19; antirheumatic agents; vaccination.
Conflict of interest statement
Competing interests: KLH reports she has received non-personal speaker’s fees from AbbVie and grant income from BMS, UCB and Pfizer, all unrelated to this manuscript; KLH is supported by the NIHR Manchester Biomedical Research Centre. LG reports personal consultant fees from AbbVie, Amgen, BMS, Biogen, Celgene, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis and UCB, and grants from Amgen, Lilly, Janssen, Pfizer, Sandoz, Sanofi and Galapagos, all unrelated to this manuscript. AS reports research grants from a consortium of 14 companies (among them AbbVie, BMS, Celltrion, Fresenius Kabi, Gilead/Galapagos, Lilly, Mylan/Viatris, Hexal, MSD, Pfizer, Roche, Samsung, Sanofi-Aventis and UCB) supporting the German RABBIT register and personal fees from lectures for AbbVie, MSD, Roche, BMS, Lilly and Pfizer, all unrelated to this manuscript. LC has not received fees or personal grants from any laboratory, but her institute works by contract for laboratories among other institutions, such as AbbVie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España, Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi-Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal and UCB Pharma. EF-M reports personal consultant fees from Boehringer Ingelheim Portugal and that LPCDR received support for specific activities: grants from AbbVie, Novartis, Janssen-Cilag, Lilly Portugal, Sanofi, Grünenthal, MSD, Celgene, Medac, Pharmakern and GAfPA; grants and non-financial support from Pfizer; and non-financial support from Grünenthal, outside the submitted work. IB reports personal consultant fees from AbbVie, Novartis, Pfizer and Janssen, all unrelated to this manuscript. JZ reports speaker fees from AbbVie, Novartis and Janssen/Johnson & Johnson, all unrelated to this manuscript. GR-C reports personal consultant fees from Eli Lilly and Novartis, all unrelated to this manuscript. JS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers: R01 AR077607, P30 AR070253 and P30 AR072577), and the R Bruce and Joan M Mickey Research Scholar Fund. JS has received research support from Amgen and Bristol Myers Squibb and performed consultancy for Bristol Myers Squibb, Gilead, Inova, Janssen and Optum, unrelated to this work. LW receives speaker’s bureau fees from Aurinia Pharma, unrelated to this manuscript. SB reports no competing interests related to this work. He reports non-branded consulting fees for AbbVie, Horizon and Novartis (all <$10 000). MGM has no competing interests related to this work. She serves as a patient consultant for BMS, BI JNJ and Aurinia (all <$10 000). RG reports no competing interests related to this work. Outside of this work she reports personal and/or speaking fees from AbbVie, Janssen, Novartis, Pfizer and Cornerstones and travel assistance from Pfizer (all <$10 000). JH reports no competing interests related to this work. He is supported by grants from the Rheumatology Research Foundation and has salary support from the Childhood Arthritis and Rheumatology Research Alliance. He has performed consulting for Novartis, Sobi and Biogen, all unrelated to this work (<$10 000). ESi reports non-financial support from Canadian Arthritis Patient Alliance, outside the submitted work. PS reports personal fees from the American College of Rheumatology/Wiley Publishing, outside the submitted work. ZW reports grant support from Bristol Myers Squibb and Principia/Sanofi and performed consultancy for Viela Bio and MedPace, outside the submitted work. His work is supported by grants from the National Institutes of Health. PMM has received consulting/speaker’s fees from AbbVie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this study. PMM is supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC). PCR reports no competing interests related to this work. Outside of this work PCR reports personal fees from AbbVie, Atom Bioscience, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Kukdong, Novartis, UCB, Roche and Pfizer; meeting attendance support from BMS, Pfizer and UCB; and grant funding from Janssen, Novartis, Pfizer and UCB Pharma (all <$10 000). JY reports no competing interests related to this work. Her work is supported by grants from the National Institutes of Health (K24 AR074534 and P30 AR070155). Outside of this work, she has received research grants or performed consulting for Gilead, BMS Foundation, Pfizer, Aurinia and AstraZeneca.
Figures
Similar articles
Hasseli R, Richter JG, Hoyer BF, Lorenz HM, Pfeil A, Regierer AC, Schmeiser T, Strangfeld A, Voll RE, Krause A, Reckert S, Gräßler A, Saar P, Kapelle A, Backhaus M, Blank N, Henes J, Osiek S, Knothe A, Hoese G, Brandt-Jürgens J, Maltzahn A, Specker C, Müller-Ladner U, Schulze-Koops H; COVID-19 task force of the German Society of Rheumatology collaborators; COVID19-Rheuma.de collaborators.RMD Open. 2023 Apr;9(2):e002998. doi: 10.1136/rmdopen-2023-002998.PMID: 37068915 Free PMC article.
Md Yusof MY, Arnold J, Saleem B, Vandevelde C, Dass S, Savic S, Vital EM, Emery P.Lancet Rheumatol. 2023 Feb;5(2):e88-e98. doi: 10.1016/S2665-9913(23)00004-8. Epub 2023 Jan 10.PMID: 36712951 Free PMC article.
Isnardi CA, Schneeberger EE, Kreimer JL, Luna PC, Echeverría C, Roberts K, de la Vega MC, Virasoro BM, Landi M, Quintana R, Exeni MED, Kogan N, Petkovic I, Pereira D, De Los Ángeles Correa M, Zelaya MD, Tissera Y, Elkin MSG, Pisoni CN, Alonso C, Cogo AK, Cosatti MA, García L, Retamozo C, de Los Ángeles Severina M, Nieto RE, Rosemffet M, Mussano E, Bertoli A, Savio VG, Cosentino V, Pons-Estel GJ.Clin Rheumatol. 2022 Oct;41(10):3199-3209. doi: 10.1007/s10067-022-06253-5. Epub 2022 Jun 28.PMID: 35760939 Free PMC article.
Paik JJ, Sparks JA, Kim AHJ.Curr Opin Pharmacol. 2022 Aug;65:102243. doi: 10.1016/j.coph.2022.102243. Epub 2022 May 2.PMID: 35636384 Free PMC article. Review.
Wang L, Wang W, Xu R, Berger NA.Best Pract Res Clin Haematol. 2022 Sep;35(3):101384. doi: 10.1016/j.beha.2022.101384. Epub 2022 Sep 29.PMID: 36494154 Free PMC article. Review.
Cited by
Joudeh AI, Lutf AQ, Mahdi S, Tran G.Vaccine. 2023 Jun 13;41(26):3801-3812. doi: 10.1016/j.vaccine.2023.05.048. Epub 2023 May 22.PMID: 37244811 Free PMC article. Review.
Hasseli R, Richter JG, Hoyer BF, Lorenz HM, Pfeil A, Regierer AC, Schmeiser T, Strangfeld A, Voll RE, Krause A, Reckert S, Gräßler A, Saar P, Kapelle A, Backhaus M, Blank N, Henes J, Osiek S, Knothe A, Hoese G, Brandt-Jürgens J, Maltzahn A, Specker C, Müller-Ladner U, Schulze-Koops H; COVID-19 task force of the German Society of Rheumatology collaborators; COVID19-Rheuma.de collaborators.RMD Open. 2023 Apr;9(2):e002998. doi: 10.1136/rmdopen-2023-002998.PMID: 37068915 Free PMC article.
Paggi R, Barbiero A, Manciulli T, Miftode A, Tilli M, Lagi F, Mencarini J, Borchi B, Pozzi M, Bartalesi F, Spinicci M, Martini L, Coppola A, Nozzoli C, Peris A, Bonizzoli M, Pieralli F, Bartoloni A, Zammarchi L.Intern Emerg Med. 2023 Apr;18(3):821-830. doi: 10.1007/s11739-023-03231-w. Epub 2023 Feb 28.PMID: 36853393 Free PMC article.
Zaccardelli A, Wallace ZS, Sparks JA.Curr Opin Rheumatol. 2023 May 1;35(3):175-184. doi: 10.1097/BOR.0000000000000930. Epub 2023 Feb 7.PMID: 36752280 Free PMC article. Review.
Md Yusof MY, Arnold J, Saleem B, Vandevelde C, Dass S, Savic S, Vital EM, Emery P.Lancet Rheumatol. 2023 Feb;5(2):e88-e98. doi: 10.1016/S2665-9913(23)00004-8. Epub 2023 Jan 10.PMID: 36712951 Free PMC article.
KMEL References
References
-
- Brown CM, Vostok J, Johnson H, et al. . Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1059–62. 10.15585/mmwr.mm7031e2 - DOI - PMC - PubMed
-
- Kearns P, Siebert S, Willicombe M. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE trial 2021. Lancet 2021.
-
- United States food and drug administration. coronavirus (COVID-19) update: FDA Authorizes additional vaccine dose for certain immunocompromised individuals. United States food and drug administration. Available: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... [Accessed 9 Sep 2021].
-
- Israeli Councils For Health . Summary of an urgent meeting on the issue of vaccines against corona for those who have been vaccinated. Israeli councils for health. Available: https://govextra.gov.il/media/30095/meeting-summary-15122020.pdf [Accessed 9 Sep 2021].
-
- COVID-19 vaccination programme information for healthcare practitioners. public health England. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploa... [Accessed 9 Sep 2021].
-
- Die wichtigsten Fragen und Antworten Zur Corona-Impfung. services Der BUNDESREGIERUNG. Available: https://www.bundesregierung.de/breg-de/themen/coronavirus/coronavirus-im... [Accessed 9 Sep 2021].
-
- Cdc COVID-19 study shows mRNA vaccines reduce risk of infection by 91 percent for fully vaccinated people, 2021. Available: https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html [Accessed 28 Oct 2021].
-
- Moor MB, Suter-Riniker F, Horn MP, et al. . Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. 10.1016/S2665-9913(21)00251-4 - DOI - PMC - PubMed
-
- Furer V, Eviatar T, Zisman D, et al. . Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 - DOI - PMC - PubMed
-
- Evusheld (formerly AZD7442) long-acting antibody combination authorised for emergency use in the US for pre-exposure prophylaxis (prevention) of COVID-19, 2021. Available: https://www.astrazeneca.com/media-centre/press-releases/2021/evusheld-lo... [Accessed 23 Jan 2022].
-
- Coronavirus (COVID-19) update: FDA Authorizes first oral antiviral for treatment of COVID-19, 2021. Available: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... [Accessed 23 Jan 2022].