MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart
Affiliations
Affiliations
- Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH; †Department of Engineering, American University of the Safat, Kuwait; ‡Department of Biomedical Informatics, Center for Biostatistics, The Ohio State University, Columbus, OH; and §Department of Emergency Medicine, Dorothy M. Davis Heart and Lung Research Institute, The Ohio State University, Columbus, OH.
Abstract
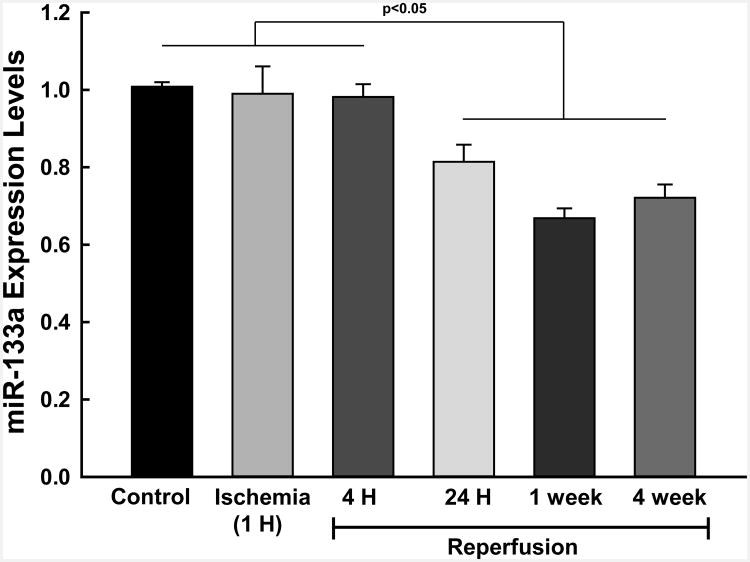
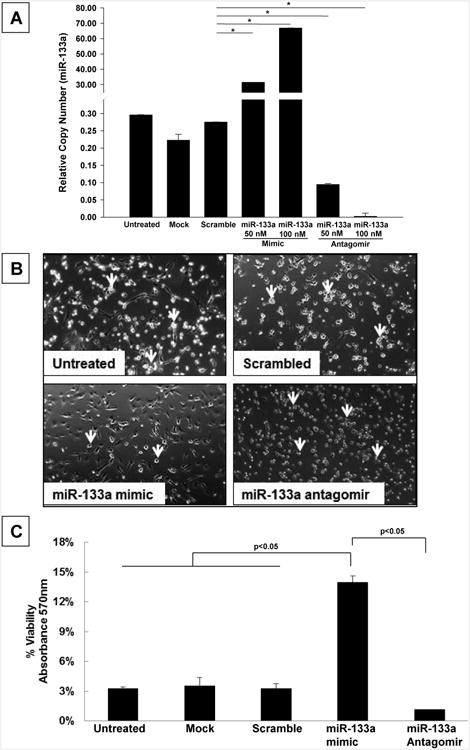
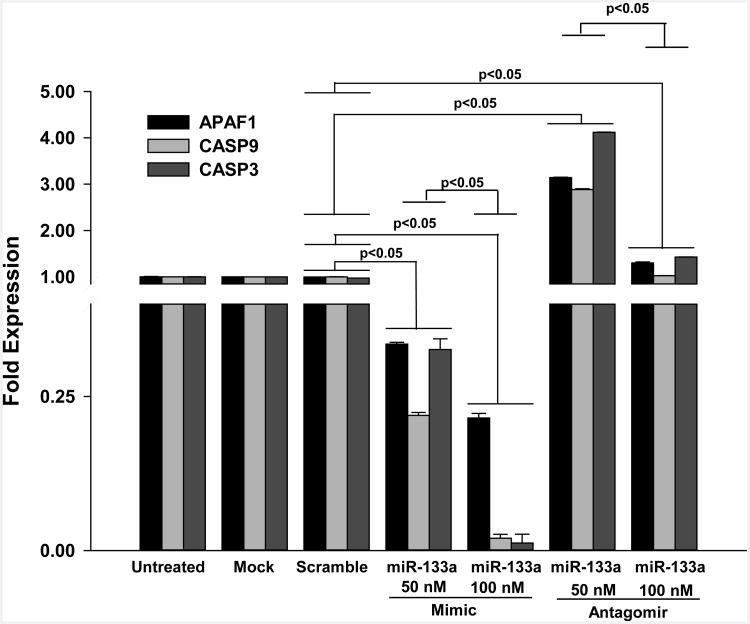
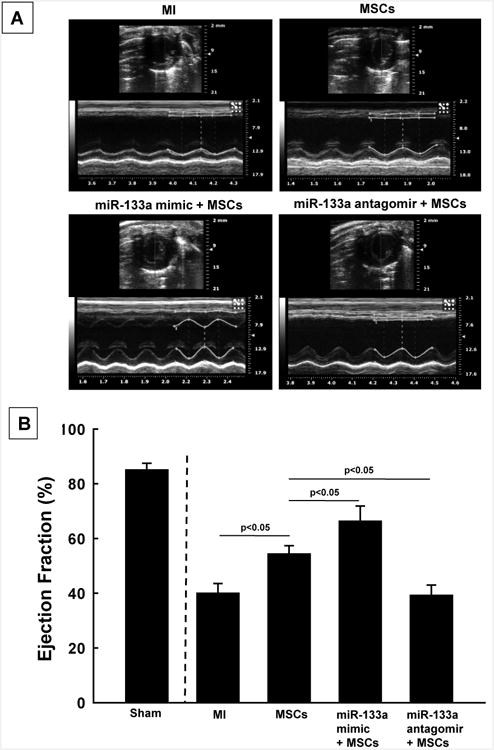
: Cardiovascular disease is the number 1 cause of morbidity and mortality in the United States. The most common manifestation of cardiovascular disease is myocardial infarction (MI), which can ultimately lead to congestive heart failure. Cell therapy (cardiomyoplasty) is a new potential therapeutic treatment alternative for the damaged heart. Recent preclinical and clinical studies have shown that mesenchymal stem cells (MSCs) are a promising cell type for cardiomyoplasty applications. However, a major limitation is the poor survival rate of transplanted stem cells in the infarcted heart. miR-133a is an abundantly expressed microRNA (miRNA) in the cardiac muscle and is downregulated in patients with MI. We hypothesized that reprogramming MSCs using miRNA mimics (double-stranded oligonucleotides) will improve survival of stem cells in the damaged heart. MSCs were transfected with miR-133a mimic and antagomirs, and the levels of miR-133a were measured by quantitative real-time polymerase chain reaction. Rat hearts were subjected to MI and MSCs transfected with miR-133a mimic or antagomir were implanted in the ischemic hearts. Four weeks after MI, cardiac function, cardiac fibrosis, miR-133a levels, and apoptosis-related genes (Apaf-1, Caspase-9, and Caspase-3) were measured in the heart. We found that transfecting MSCs with miR-133a mimic improves survival of MSCs as determined by the MTT assay. Similarly, transplantation of miR-133a mimic transfected MSCs in rat hearts subjected to MI led to a significant increase in cell engraftment, cardiac function, and decreased fibrosis when compared with MSCs only or MI groups. At the molecular level, quantitative real-time polymerase chain reaction data demonstrated a significant decrease in expression of the proapoptotic genes; Apaf-1, caspase-9, and caspase-3 in the miR-133a mimic transplanted group. Furthermore, luciferase reporter assay confirmed that miR-133a is a direct target for Apaf-1. Overall, bioengineering of stem cells through miRNAs manipulation could potentially improve the therapeutic outcome of patients undergoing stem cell transplantation for MI.
Conflict of interest statement
Disclosure and Conflicts of Interest: None.
Figures
Similar articles
Chen Y, Zhao Y, Chen W, Xie L, Zhao ZA, Yang J, Chen Y, Lei W, Shen Z.Stem Cell Res Ther. 2017 Nov 25;8(1):268. doi: 10.1186/s13287-017-0722-z.PMID: 29178928 Free PMC article.
Zhang Y, Lei W, Yan W, Li X, Wang X, Zhao Z, Hui J, Shen Z, Yang J.Stem Cell Res Ther. 2016 Apr 22;7(1):61. doi: 10.1186/s13287-016-0318-z.PMID: 27103465 Free PMC article.
Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu Y, Su T, Sun D, Li W, Wang H, Liu B, Cao F.Basic Res Cardiol. 2014 Jul;109(4):417. doi: 10.1007/s00395-014-0417-x. Epub 2014 May 22.PMID: 24847908
MicroRNA-133a and Myocardial Infarction.
Xiao Y, Zhao J, Tuazon JP, Borlongan CV, Yu G.Cell Transplant. 2019 Jul;28(7):831-838. doi: 10.1177/0963689719843806. Epub 2019 Apr 14.PMID: 30983393 Free PMC article. Review.
Chistiakov DA, Orekhov AN, Bobryshev YV.J Mol Cell Cardiol. 2016 May;94:107-121. doi: 10.1016/j.yjmcc.2016.03.015. Epub 2016 Apr 4.PMID: 27056419 Review.
Cited by
Huang X, Xu X, Wang C, Wang Y, Yang Y, Yao T, Bai R, Pei X, Bai F, Li P.Front Neurosci. 2023 Apr 18;17:1145307. doi: 10.3389/fnins.2023.1145307. eCollection 2023.PMID: 37144089 Free PMC article.
The Role of ncRNAs in Cardiac Infarction and Regeneration.
Caño-Carrillo S, Lozano-Velasco E, Castillo-Casas JM, Sánchez-Fernández C, Franco D.J Cardiovasc Dev Dis. 2023 Mar 15;10(3):123. doi: 10.3390/jcdd10030123.PMID: 36975887 Free PMC article. Review.
Xiao R, Zhou M, Wang P, Zeng B, Wu L, Hu Z, Liang D.Int J Mol Sci. 2022 Aug 16;23(16):9176. doi: 10.3390/ijms23169176.PMID: 36012442 Free PMC article.
Functional Role of microRNAs in Regulating Cardiomyocyte Death.
Kansakar U, Varzideh F, Mone P, Jankauskas SS, Santulli G.Cells. 2022 Mar 12;11(6):983. doi: 10.3390/cells11060983.PMID: 35326433 Free PMC article. Review.
Tian T, Li F, Chen R, Wang Z, Su X, Yang C.Front Cell Dev Biol. 2022 Jan 19;9:779524. doi: 10.3389/fcell.2021.779524. eCollection 2021.PMID: 35127703 Free PMC article.