Pharmacokinetic Characterisation and Comparison of Bioavailability of Intranasal Fentanyl, Transmucosal, and Intravenous Administration through a Three-Way Crossover Study in 24 Healthy Volunteers
Affiliations
Affiliations
- Department of Anaesthesia and Intensive Care, University Hospital Marburg, Marburg, Germany.
- Division of Medicine, University College London, London, UK.
- Department of Mathematics and Natural Science, Gulf University of Science and Technology, Mubarak Al-Abdullah, Kuwait.
- Royal Free London Hospital, University College London, London, UK.
- Department of Anaesthesiology Intensive Care Medicine Emergency Medicine and Pain Therapy, RKH Kliniken-Hospital Ludwigsburg, Ludwigsburg, Germany.
- Department of Anaesthesiology Intensive Care Medicine Emergency Medicine and Pain Therapy, Klinikum Kassel GmbH, Kassel, Germany.
Abstract
Background: For more than 60 years, the synthetic opioid fentanyl has been widely used in anaesthesia and analgesia. While the intravenous formulation is primarily used for general anaesthesia and intensive care settings, the drug's high lipophilic properties also allow various noninvasive routes of administration. Published data suggest that intranasal administration is also attractive for use as intranasal patient-controlled analgesia (PCA). A newly developed intranasal fentanyl formulation containing 47 μg fentanyl, intravenous fentanyl, and oral transmucosal fentanyl citrate were characterised, and bioavailability was compared to assess the suitability of the intranasal formulation for an intranasal PCA product.
Methods: 27 healthy volunteers were enrolled in a single-centre, open-label, randomised (order of treatments), single-dose study in a three-period crossover design. The pharmacokinetics of one intranasal puff of fentanyl formulation (47 μg, 140 mL per puff), one short intravenous infusion of 50 μg fentanyl, and one lozenge with an integrated applicator (200 μg fentanyl) were studied, and bioavailability was calculated. Blood samples were collected over 12 hours, and plasma concentrations of fentanyl were determined by HPLC with MS/MS detection.
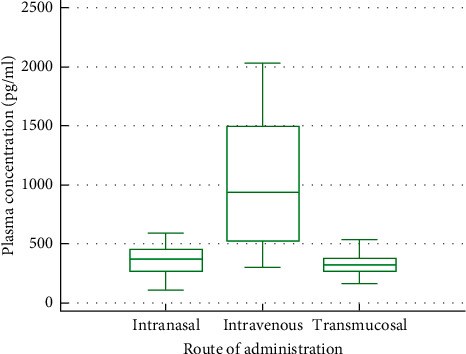
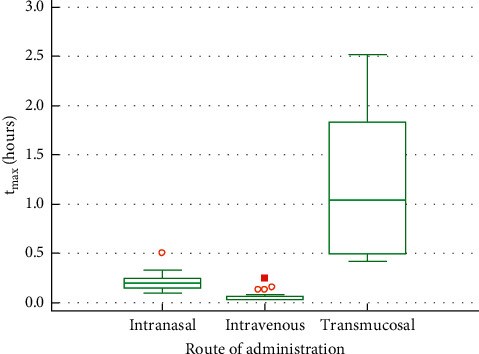
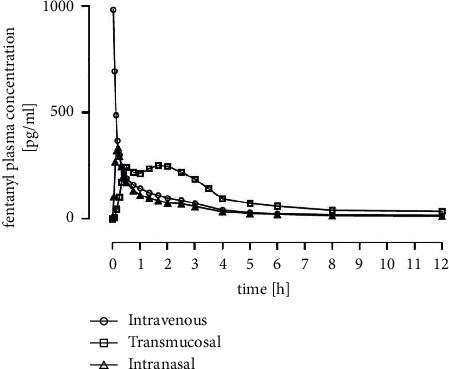
Results: 24 volunteers completed the study. The geometric mean of AUC0-tlast was the highest with oral transmucosal administration (1106 h ∗ pg/ml, CV% = 32.86), followed by intravenous (672 h ∗ pg/ml, CV% = 32.18) and intranasal administration (515 h ∗ pg/ml, CV% = 30.10). C max was 886 pg/ml (CV% = 59.38) for intravenous, 338 pg/ml (CV% = 45.61) for intranasal, and 310 pg/ml (CV% = 29.58) for oral transmucosal administration. t max was shortest for intravenous administration (0.06 h, SD = 0.056), followed by intranasal (0.21 h, SD = 0.078) and oral transmucosal administration (1.20 h, SD = 0.763). Dose-adjusted absolute bioavailability was determined to be 74.70% for the intranasal formulation and 41.25% for the oral transmucosal product. In total, 38 adverse events (AEs) occurred. Fourteen AEs were potentially related to the investigational items. No serious AE occurred.
Conclusion: Pharmacokinetic parameters and bioavailability of the investigated intranasal fentanyl indicated suitability for its intended use as an intranasal PCA option.
Conflict of interest statement
SNH and LE are co-inventors of the aforementioned intranasal PCA device under development. The other co-authors do not have anything to declare.
Figures
Similar articles
Darwish M, Tempero K, Kirby M, Thompson J.Clin Ther. 2006 May;28(5):715-24. doi: 10.1016/j.clinthera.2006.05.016.PMID: 16861093 Clinical Trial.
Parikh N, Goskonda V, Chavan A, Dillaha L.Clin Ther. 2013 Mar;35(3):236-43. doi: 10.1016/j.clinthera.2013.02.017.PMID: 23497761 Clinical Trial.
Nave R, Schmitt H, Popper L.Drug Deliv. 2013 Jun-Jul;20(5):216-23. doi: 10.3109/10717544.2012.762435. Epub 2013 May 8.PMID: 23650968 Clinical Trial.
Opioids for the management of breakthrough pain in cancer patients.
Zeppetella G, Davies AN.Cochrane Database Syst Rev. 2013 Oct 21;(10):CD004311. doi: 10.1002/14651858.CD004311.pub3.PMID: 24142465 Updated. Review.
Pharmacokinetics of non-intravenous formulations of fentanyl.
Lötsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G.Clin Pharmacokinet. 2013 Jan;52(1):23-36. doi: 10.1007/s40262-012-0016-7.PMID: 23100195 Review.
Cited by
Shetabi H, Koohi H.Anesth Pain Med. 2022 Nov 8;12(4):e130452. doi: 10.5812/aapm-130452. eCollection 2022 Aug.PMID: 36937086 Free PMC article.
Mangoni AA, Ceruti T, Frapolli R, Russo M, Fichera S, Zucchetti M, Tommasi S.Molecules. 2022 Feb 2;27(3):1017. doi: 10.3390/molecules27031017.PMID: 35164277 Free PMC article.
KMEL References
References
-
- Labroo R. B., Paine M. F., Thummel K. E., Kharasch E. D. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metabolism and Disposition: The Biological Fate of Chemicals . 1997;25:1072–1080. - PubMed
-
- Viscusi E. R., Reynolds L., Tait S., Melson T., Atkinson L. E. An iontophoretic fentanyl patient-activated analgesic delivery system for postoperative pain: a double-blind, placebo-controlled trial. Anesthesia & Analgesia . 2006;102(1):188–194. doi: 10.1213/01.ane.0000183649.58483.77. - DOI - PubMed
-
- Lim S. C., Paech M. J., Sunderland V. B., Roberts M. J., Banks S. L., Rucklidge M. W. Pharmacokinetics of nasal fentanyl. Journal of Pharmacy Practice and Research . 2003;33(1):59–64. doi: 10.1002/jppr200333159. - DOI
-
- Nardi-Hiebl S., Meuser T., Geldner G., Schneider J., Koch T., Chappell Eberhart L. H. Quo vadis OPS 8-919? Eine Analyse der Kodierungen und die Bedeutung für den klinischen Alltag. Pain Therapy . 2021;62:146–156. doi: 10.19224/ai2021.146. - DOI
-
- Eberhart L., Nardi-Hiebl S., Kubitz N., Koch T., Grond S., Schreder H. Prozesskostenanalyse in der postoperativen Schmerztherapie—vergleich der iv PCA mit einem nicht-invasiven, iontophoretischen, patientenaktivierten, transdermalen System (iPATS) Anästhesiologie & Intensivmedizin . 2009;50:p. 677.
-
- Thomas Meuser M. D., Stefan Nardi-Hiebl Ms, Leopold Eberhart M. D., Matthias Paul M. D., Böttger R., Jörg Reutershan M. D. Staff time requirements for postoperative pain management: comparison of sufentanil sublingual tablet system and intravenous patient-controlled analgesia. Journal of Opioid Management . 2020;16:33–39. - PubMed
-
- Eberhart L., Jackl I., Nardi-Hiebl S. Nasal Applicator . 2015. US9033939B2.
-
- State Chamber of Physicians of Thuringia. Germany. Reference ZIE/1045/06/111. Protocol registration number 05ct148fe n.d.
-
- European Union Drug Regulating Authorities Clinical Trials Database. Nr. 003034-17 n.d.
-
- Veldhorst-Janssen N. M. L., Fiddelers A. A. A., van der Kuy P.-H. M., et al. Pharmacokinetics, analgesic effect, and tolerability of a single preprocedural dose of intranasal fentanyl in patients undergoing drain removal after breast reduction or augmentation surgery: a prospective, randomized, double-blind, placebo-controlled study. Clinical Therapeutics . 2010;32(7):1427–1436. doi: 10.1016/j.clinthera.2010.07.001. - DOI - PubMed
-
- Kuip E. J. M., Zandvliet M. L., Koolen S. L. W., Mathijssen R. H. J., van der Rijt C. C. D. A review of factors explaining variability in fentanyl pharmacokinetics; focus on implications for cancer patients. British Journal of Clinical Pharmacology . 2017;83(2):294–313. doi: 10.1111/bcp.13129. - DOI - PMC - PubMed
-
- Janknegt R., van den Beuken M., Schiere S., et al. Rapid acting fentanyl formulations in breakthrough pain in cancer. Drug selection by means of the System of Objectified Judgement Analysis. The European Journal of Hospital Pharmacy: Science and Practice . 2018;25:p. e2. doi: 10.1136/ejhpharm-2016-001127. - DOI - PMC - PubMed