Deintensification of Treatment With Sulfonylurea and Insulin After Severe Hypoglycemia Among Older Adults With Diabetes
Affiliations
Affiliations
- Department of Medicine, Division of Endocrinology, Duke University, Durham, North Carolina.
- Durham Veterans Affairs Center of Innovation to Accelerate Discovery and Practice Transformation, Durham, North Carolina.
- Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill.
- Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill.
- Division of Epidemiology, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland.
- Department of Medicine, Division of Geriatrics and Center for Aging and Health, University of North Carolina at Chapel Hill, Chapel Hill.
- Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill.
- Center of Health Equity Research and Promotion, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania.
- Department of Medicine, Division of Endocrinology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill.
Abstract
Importance: Practice guidelines recommend deintensification of hypoglycemic agents among older adults with diabetes who are at high risk of hypoglycemia, yet real-world treatment deintensification practices are not well characterized.
Objective: To examine the incidence of sulfonylurea and insulin deintensification after a hypoglycemia-associated emergency department (ED) visit or hospitalization among older adults with diabetes and to identify factors associated with deintensification of treatment.
Design, setting, and participants: This retrospective cohort study included a random sample of 20% of nationwide fee-for-service US Medicare beneficiaries aged 65 years and older with concurrent Medicare parts A, B, and D coverage between January 1, 2007, and December 31, 2017. Individuals with diabetes who had at least 1 hypoglycemia-associated ED visit or hospitalization were included. Data were analyzed from August 1, 2020, to August 1, 2021.
Exposures: Baseline medication for the treatment of diabetes (sulfonylurea, insulin, or both).
Main outcomes and measures: Incidence of treatment deintensification (yes or no) in the 100 days after a severe hypoglycemic episode requiring an ED visit or hospitalization, with treatment deintensification defined as (1) a decrease in sulfonylurea dose, (2) a change from long-acting to short-acting sulfonylurea (glipizide), (3) discontinuation of sulfonylurea, or (4) discontinuation of insulin based on pharmacy dispensing claims.
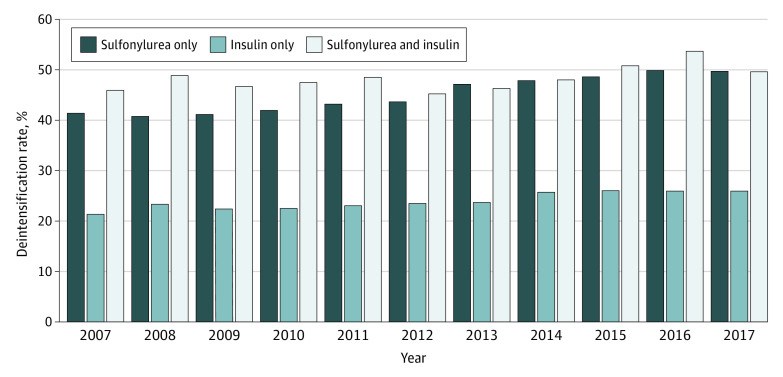
Results: Among 76 278 distinct Medicare beneficiaries who had a hypoglycemia-associated ED visit or hospitalization, the mean (SD) age was 76.6 (7.6) years. Of 106 293 total hypoglycemic episodes requiring hospital attention, 69 084 (65.0%) occurred among women, 26 056 (24.5%) among Black individuals; 4761 (4.5%) among Hispanic individuals; 69 704 (65.6%) among White individuals; and 5772 (5.4%) among individuals of other races and ethnicities (comprising Asian, North American Native, unknown race or ethnicity, and unspecified race or ethnicity). A total of 32 074 episodes (30.2%) occurred among those receiving sulfonylurea only, 60 350 (56.8%) occurred among those receiving insulin only, and 13 869 (13.0%) occurred among those receiving both sulfonylurea and insulin. Treatment deintensification rates were highest among individuals receiving both sulfonylurea and insulin therapies at the time of their hypoglycemic episode (6677 episodes [48.1%]), followed by individuals receiving sulfonylurea only (14 192 episodes [44.2%]) and insulin only (14 495 episodes [24.0%]). Treatment deintensification rates increased between 2007 and 2017 (sulfonylurea only: from 41.4% to 49.7%; P < .001 for trend; insulin only: from 21.3% to 25.9%; P < .001 for trend; sulfonylurea and insulin: from 45.9% to 49.6%; P = .005 for trend). Lower socioeconomic status (as indicated by the receipt of low-income subsidies) was associated with lower odds of deintensification, regardless of baseline hypoglycemic regimen (sulfonylurea only: adjusted odds ratio [AOR], 0.74 [95% CI, 0.70-0.78]; insulin only: AOR, 0.71 [95% CI, 0.68-0.75]; sulfonylurea and insulin: AOR, 0.72 [95% CI, 0.66-0.78]). A number of patient factors were associated with higher odds of treatment deintensification: higher frailty (eg, ≥40% probability of needing assistance with activities of daily living among those receiving sulfonylurea and insulin: AOR, 1.50; 95% CI, 1.32-1.71), chronic kidney disease (eg, sulfonylurea and insulin: AOR, 1.29; 95% CI, 1.19-1.40), a history of falls (eg, sulfonylurea and insulin: AOR, 1.20; 95% CI, 1.09-1.33), and depression (eg, sulfonylurea and insulin: AOR, 1.11; 95% CI, 1.02-1.20).
Conclusions and relevance: In this cohort study, deintensification of sulfonylurea and/or insulin therapy within 100 days after a hypoglycemia-associated ED visit or hospitalization occurred in fewer than 50% of older adults with diabetes; however, these deintensification rates may be increasing over time, and deintensification of insulin was likely underestimated because of challenges in capturing changes to insulin dosing using administrative claims data. These results suggest that greater efforts are needed to identify individuals at high risk of hypoglycemia to encourage appropriate treatment deintensification in accordance with current evidence.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Kahkoska reported receiving travel support from Novo Nordisk outside the submitted work. Dr Stürmer reported receiving grants from Novo Nordisk; salary support from the Center for Pharmacoepidemiology (comprising AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Takeda Pharmaceutical, and UCB BioSciences); a generous donation from Dr Nancy Dreyer (via the Department of Epidemiology, University of North Carolina at Chapel Hill); and owning stock in Novartis, Novo Nordisk, and Roche outside the submitted work. Dr Buse reported receiving grants from the American Diabetes Association, AstraZeneca, Dexcom, Eli Lilly and Company, Intarcia Therapeutics, Johnson & Johnson, the Juvenile Diabetes Research Foundation, Lexicon Pharmaceuticals, the National Institutes of Health, NovaTarg Therapeutics, Novo Nordisk, the Patient-Centered Outcomes Research Institute, Sanofi, Theracos, Tolerion, and vTv Therapeutics; consulting fees and travel support (via the University of North Carolina at Chapel Hill) from Adocia, AstraZeneca, Eli Lilly and Company, Intarcia Therapeutics, MannKind Corp, Novo Nordisk, Sanofi, Senseonics Holdings, and vTv Therapeutics; personal fees from Anji Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Cirius Therapeutics, Dasman Diabetes Institute (Kuwait), Eli Lilly and Company, Fortress Biotech, Glyscend, Janssen Pharmaceuticals, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Stability Health, and Zealand Pharma; and owning stock in Mellitus Health, Pendulum Therapeutics, PhaseBio Pharmaceuticals, Praetego, and Stability Health outside the submitted work. No other disclosures were reported.
Figures
Similar articles
McCoy RG, Lipska KJ, Van Houten HK, Shah ND.JAMA Netw Open. 2020 Jan 3;3(1):e1919099. doi: 10.1001/jamanetworkopen.2019.19099.PMID: 31922562 Free PMC article.
Romley JA, Gong C, Jena AB, Goldman DP, Williams B, Peters A.BMJ. 2015 Dec 7;351:h6223. doi: 10.1136/bmj.h6223.PMID: 26643108 Free PMC article.
Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas.
Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS.JAMA Intern Med. 2014 Oct;174(10):1605-12. doi: 10.1001/jamainternmed.2014.3293.PMID: 25179404 Free PMC article.
Schopman JE, Simon AC, Hoefnagel SJ, Hoekstra JB, Scholten RJ, Holleman F.Diabetes Metab Res Rev. 2014 Jan;30(1):11-22. doi: 10.1002/dmrr.2470.PMID: 24030920 Review.
Early insulin use in type 2 diabetes: what are the cons?
Chiasson JL.Diabetes Care. 2009 Nov;32 Suppl 2(Suppl 2):S270-4. doi: 10.2337/dc09-S321.PMID: 19875563 Free PMC article. Review. No abstract available.
Cited by
Alexopoulos AS, Crowley MJ, Kahkoska AR.Diabetes Care. 2023 Jun 1;46(6):1137-1139. doi: 10.2337/dci23-0017.PMID: 37220268 Free PMC article. No abstract available.
KMEL References
References
-
- McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control, and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA Netw Open. 2020;3(1):e1919099. doi:10.1001/jamanetworkopen.2019.19099 - DOI - PMC - PubMed
-
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi:10.1016/j.ijsu.2014.07.013 - DOI - PubMed
-
- Vue MH, Setter SM. Drug-induced glucose alterations, part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177. doi:10.2337/diaspect.24.3.171 - DOI