Reticular dysgenesis: international survey on clinical presentation, transplantation, and outcome
Manfred Hoenig 1, Chantal Lagresle-Peyrou 2, Ulrich Pannicke 3 4, Luigi D Notarangelo 5, Fulvio Porta 6, Andrew R Gennery 7 8, Mary Slatter 7 8, Morton J Cowan 9, Polina Stepensky 10, Hamoud Al-Mousa 11, Daifulah Al-Zahrani 12, Sung-Yun Pai 13 14, Waleed Al Herz 15, Hubert B Gaspar 16 17, Paul Veys 16 17, Koichi Oshima 18, Kohsuke Imai 19, Hiromasa Yabe 20, Lenora M Noroski 21, Nico M Wulffraat 22, Karl-Walter Sykora 23, Pere Soler-Palacin 24, Hideki Muramatsu 25, Mariam Al Hilali 26, Despina Moshous 27, Klaus-Michael Debatin 1, Catharina Schuetz 1, Eva-Maria Jacobsen 1, Ansgar S Schulz 1, Klaus Schwarz 3 4, Alain Fischer 27, Wilhelm Friedrich 1, Marina Cavazzana 2; European Society for Blood and Marrow Transplantation (EBMT) Inborn Errors Working Party
Affiliations
Affiliations
- Department of Pediatrics, University Medical Center Ulm, Ulm, Germany.
- Biotherapy Clinical Investigation Center, Hôpital Necker Enfants Malades, Paris, France.
- Institute for Transfusion Medicine, University of Ulm, Ulm, Germany.
- Institute for Clinical Transfusion Medicine and Immunogenetics Ulm, German Red Cross Blood Service Baden-Wuerttemberg - Hessen, Ulm, Germany.
- Division of Immunology, Boston Children's Hospital, Boston, MA.
- Department of Pediatrics, University Hospital, Brescia, Italy.
- Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom.
- Department of Paediatric Immunology and Haematopoietic Stem Cell Transplant (HSCT), Great North Children´s Hospital, Newcastle upon Tyne, United Kingdom.
- Division of Allergy, Immunology and Bone Marrow Transplant (BMT), UCSF Benioff Children´s Hospital, San Francisco, CA.
- Pediatric Hematology-Oncology, Hadassah University Hospital, Jerusalem, Israel.
- Section of Pediatric Allergy/Immunology, Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
- Department of Pediatrics, Allergy, Immunology and BMT, King Saud Bin Abdulaziz University for Health Sciences, King Abdulaziz Medical City, Jeddah, Saudi Arabia.
- Division of Hematology-Oncology, Boston Children's Hospital, Boston, MA.
- Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston, MA.
- Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait.
- UCL Great Ormond Street Institute of Child Health, London, United Kingdom.
- Great Ormond Street Hospital for Children NHS Trust, London, United Kingdom.
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan.
- Department of Community Pediatrics, Perinatal and Maternal Medicine, Tokyo Medical and Dental University, Tokyo, Japan.
- Department of Cell Transplantation and Regenerative Medicine, Tokai University School of Medicine, Isahara, Japan.
- Department of Allergy and Immunology, Section of Immunology, Allergy and Rheumatology, Texas Children's Hospital, Baylor College of Medicine, Houston, TX.
- Department of Pediatric Immunology, University Medical Center Utrecht, Utrecht, The Netherlands.
- Department of Pediatric Hematology and Oncology, Hannover Medical School, Hannover, Germany.
- Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d´Hebron, Barcelona, Spain.
- Department of Pediatrics, Nagoya University Graduate School of Medicine, Nagoya, Japan.
- Department of Pediatrics, Prince Sultan Military Medical City, Riyadh, Saudi Arabia; and.
- Paediatric Immunology, Haematology and Rheumatology, Assistance Publique - Hôpitaux de Paris, Hôpital Necker-Enfants Malades, Paris, France.
Abstract
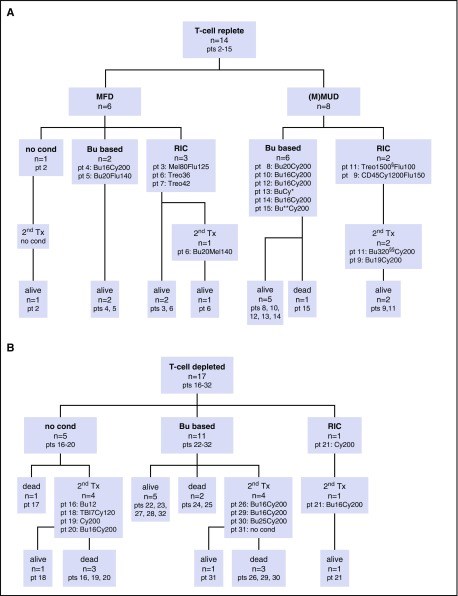
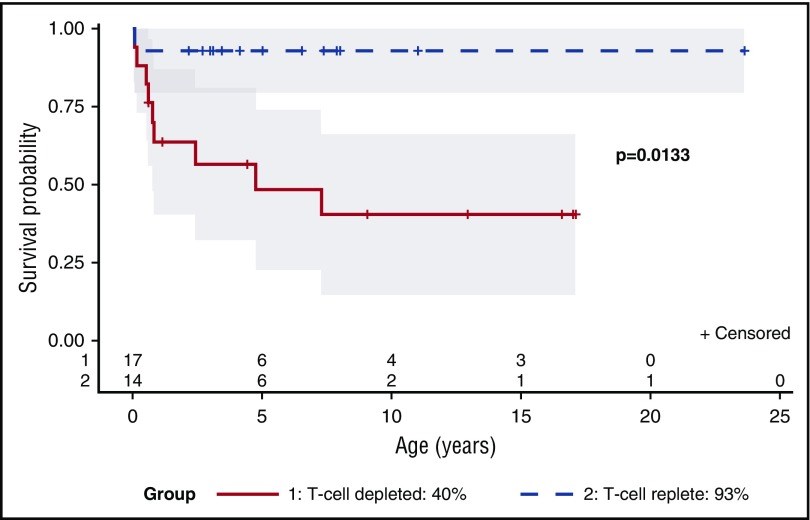
Reticular dysgenesis (RD) is a rare congenital disorder defined clinically by the combination of severe combined immunodeficiency (SCID), agranulocytosis, and sensorineural deafness. Mutations in the gene encoding adenylate kinase 2 were identified to cause the disorder. Hematopoietic stem cell transplantation (HSCT) is the only option to cure this otherwise fatal disease. Retrospective data on clinical presentation, genetics, and outcome of HSCT were collected from centers in Europe, Asia, and North America for a total of 32 patients born between 1982 and 2011. Age at presentation was <4 weeks in 30 of 32 patients (94%). Grafts originated from mismatched family donors in 17 patients (55%), from matched family donors in 6 patients (19%), and from unrelated marrow or umbilical cord blood donors in 8 patients (26%). Thirteen patients received secondary or tertiary transplants. After transplantation, 21 of 31 patients were reported alive at a mean follow-up of 7.9 years (range: 0.6-23.6 years). All patients who died beyond 6 months after HSCT had persistent or recurrent agranulocytosis due to failure of donor myeloid engraftment. In the absence of conditioning, HSCT was ineffective to overcome agranulocytosis, and inclusion of myeloablative components in the conditioning regimens was required to achieve stable lymphomyeloid engraftment. In comparison with other SCID entities, considerable differences were noted regarding age at presentation, onset, and type of infectious complications, as well as the requirement of conditioning prior to HSCT. Although long-term survival is possible in the presence of mixed chimerism, high-level donor myeloid engraftment should be targeted to avoid posttransplant neutropenia.
Figures
Similar articles
Ahmed N, Leung KS, Rosenblatt H, Bollard CM, Gottschalk S, Myers GD, Carrum G, Heslop HE, Brenner MK, Krance RA.Biol Blood Marrow Transplant. 2008 Nov;14(11):1298-304. doi: 10.1016/j.bbmt.2008.09.003.PMID: 18940685
Recent advances in understanding the pathogenesis and management of reticular dysgenesis.
Hoenig M, Pannicke U, Gaspar HB, Schwarz K.Br J Haematol. 2018 Mar;180(5):644-653. doi: 10.1111/bjh.15045. Epub 2017 Dec 21.PMID: 29270983 Review.
Lankester AC, Neven B, Mahlaoui N, von Asmuth EGJ, Courteille V, Alligon M, Albert MH, Serra IB, Bader P, Balashov D, Beier R, Bertrand Y, Blanche S, Bordon V, Bredius RG, Cant A, Cavazzana M, Diaz-de-Heredia C, Dogu F, Ehlert K, Entz-Werle N, Fasth A, Ferrua F, Ferster A, Formankova R, Friedrich W, Gonzalez-Vicent M, Gozdzik J, Güngör T, Hoenig M, Ikinciogullari A, Kalwak K, Kansoy S, Kupesiz A, Lanfranchi A, Lindemans CA, Meisel R, Michel G, Miranda NAA, Moraleda J, Moshous D, Pichler H, Rao K, Sedlacek P, Slatter M, Soncini E, Speckmann C, Sundin M, Toren A, Vettenranta K, Worth A, Yeşilipek MA, Zecca M, Porta F, Schulz A, Veys P, Fischer A, Gennery AR.J Allergy Clin Immunol. 2022 May;149(5):1744-1754.e8. doi: 10.1016/j.jaci.2021.10.017. Epub 2021 Oct 27.PMID: 34718043
Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins AM, Gibson B, Sedlacek P, Krol L, Strahm B, Zaidman I, Kalwak K, Talano JA, Woolfrey A, Fraser C, Meyts I, Müller I, Wachowiak J, Bernardo ME, Veys P, Sykora KW, Gennery AR, Slatter M.Blood. 2016 Jul 21;128(3):440-8. doi: 10.1182/blood-2016-03-704015. Epub 2016 May 23.PMID: 27216217 Free PMC article. Clinical Trial.
Heimall J, Puck J, Buckley R, Fleisher TA, Gennery AR, Neven B, Slatter M, Haddad E, Notarangelo LD, Baker KS, Dietz AC, Duncan C, Pulsipher MA, Cowan MJ.Biol Blood Marrow Transplant. 2017 Mar;23(3):379-387. doi: 10.1016/j.bbmt.2016.12.619. Epub 2017 Jan 6.PMID: 28068510 Free PMC article. Review.
Cited by
Lankester AC, Albert MH, Booth C, Gennery AR, Güngör T, Hönig M, Morris EC, Moshous D, Neven B, Schulz A, Slatter M, Veys P; Inborn Errors Working Party of the European Society for Blood and Marrow Transplantation and the European Society for Immune Deficiencies, and European Reference Network on Rare Primary Immunodeficiency Autoinflammatory Autoimmune diseases (RITA).Bone Marrow Transplant. 2021 Sep;56(9):2052-2062. doi: 10.1038/s41409-021-01378-8. Epub 2021 Jul 5.PMID: 34226669 Free PMC article. Review. No abstract available.
Adenylate kinase 2 expression and addiction in T-ALL.
Maslah N, Latiri M, Asnafi V, Féroul M, Bedjaoui N, Steimlé T, Six E, Verhoyen E, Macintyre E, Lagresle-Peyrou C, Lhermitte L, Andrieu GP.Blood Adv. 2021 Feb 9;5(3):700-710. doi: 10.1182/bloodadvances.2020002700.PMID: 33560378 Free PMC article.
Rissone A, Jimenez E, Bishop K, Carrington B, Slevin C, Wincovitch SM, Sood R, Candotti F, Burgess SM.Dis Model Mech. 2019 Dec 20;12(12):dmm040170. doi: 10.1242/dmm.040170.PMID: 31727854 Free PMC article.
Ghaloul-Gonzalez L, Mohsen AW, Karunanidhi A, Seminotti B, Chong H, Madan-Khetarpal S, Sebastian J, Vockley CW, Reyes-Múgica M, Vander Lugt MT, Vockley J.Sci Rep. 2019 Oct 31;9(1):15739. doi: 10.1038/s41598-019-51922-2.PMID: 31673062 Free PMC article.
Modeling hematopoietic disorders in zebrafish.
Konantz M, Schürch C, Hanns P, Müller JS, Sauteur L, Lengerke C.Dis Model Mech. 2019 Sep 6;12(9):dmm040360. doi: 10.1242/dmm.040360.PMID: 31519693 Free PMC article. Review.
![Figure 1. May-Grunwald-Giemsa stain of a bone marrow aspirate from a patient with RD (original magnification ×1000, immersion oil, Zeiss Axio Imager M1, AxioCam MRc [Carl Zeiss Microscopy, Jena, Germany]) showing immature lymphoid cells (hematogones) with slim cytoplasm as well as myeloblasts and a dysplastic promyelocyte with nuclear and cytoplasmic vacuoles. In the work-up of the bone marrow with immunostaining and flow cytometry, immature lymphocytes were identified as polyclonal B-cell precursors (flow cytometry of the bone marrow revealed a population of 46% with markers of pre–B cells CD10+/CD19+/cyIgM+/TdT+/CD34–; clonality was tested in a multiplex polymerase chain reaction for the rearranged B-cell receptor heavy chains [data not shown]).](/Images/Figures/19_5239.jpeg)