Rubella Virus Infected Macrophages and Neutrophils Define Patterns of Granulomatous Inflammation in Inborn and Acquired Errors of Immunity
Ludmila Perelygina 1, Raeesa Faisthalab 1, Emily Abernathy 1, Min-Hsin Chen 1, LiJuan Hao 1, Lionel Bercovitch 2, Diana K Bayer 3, Lenora M Noroski 4, Michael T Lam 4, Maria Pia Cicalese 5, Waleed Al-Herz 6 7, Arti Nanda 8, Joud Hajjar 4, Koen Vanden Driessche 9, Shari Schroven 9, Julie Leysen 10, Misha Rosenbach 11, Philipp Peters 12, Johannes Raedler 12, Michael H Albert 12, Roshini S Abraham 13, Hemalatha G Rangarjan 14, David Buchbinder 15 16, Lisa Kobrynski 17, Anne Pham-Huy 18, Julie Dhossche 19, Charlotte Cunningham Rundles 20, Anna K Meyer 21, Amy Theos 22, T Prescott Atkinson 23, Amy Musiek 24, Mehdi Adeli 25, Ute Derichs 26, Christoph Walz 27, Renate Krüger 28, Horst von Bernuth 28 29 30 31, Christoph Klein 12, Joseph Icenogle 1, Fabian Hauck 12, Kathleen E Sullivan 32
Affiliations
Affiliations
- Centers for Disease Control and Prevention, Division of Viral Diseases, Atlanta, GA, United States.
- Department of Dermatology, Hasbro Children's Hospital and Warren Alpert Medical School of Brown University, Providence, RI, United States.
- Department of Pediatrics, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States.
- Department of Pediatrics, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, United States.
- Pediatric Immunohematology and Bone Marrow Transplantation Unit and San Raffaele Telethon Institute for Gene Therapy (SR-TIGET), Istituto di Ricovero e Cura a Carattere Scientifico (National Institute for Research and Treatment) (IRCCS) San Raffaele Scientific Institute, Milan, Italy.
- Department of Pediatrics, Kuwait University, Kuwait City, Kuwait.
- Allergy and Clinical Immunology Unit, Department of Pediatrics, Al-Sabah Hospital, Kuwait City, Kuwait.
- Pediatric Dermatology Unit, As'ad Al-Hamad Dermatology Center, Al-sabah Hospital, Kuwait City, Kuwait.
- Department of Pediatrics, Queen Mathilde Mother and Child Centre, Antwerp University Hospital, Antwerp, Belgium.
- Department of Dermatology, Queen Mathilde Mother and Child Centre, Antwerp University Hospital, Antwerp, Belgium.
- Department of Dermatology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States.
- Department of Pediatrics, Dr. von Hauner Children's Hospital, University Hospital, Ludwig-Maximilians-Universität München, Munich, Germany.
- Department of Pathology and Laboratory Medicine, Nationwide Children's Hospital, Columbus, OH, United States.
- Department of Hematology, Oncology, Blood and Marrow Transplant, Nationwide Children's Hospital, Columbus, OH, United States.
- Department of Hematology, Children's Hospital of Orange County, Orange, CA, United States.
- Department of Pediatrics, University of California at Irvine, Orange, CA, United States.
- Allergy/Immunology Section, Emory University, Atlanta, GA, United States.
- Department of Pediatrics, University of Ottawa and Children's Hospital of Eastern Ontario, Ottawa, ON, Canada.
- Department of Dermatology, Oregon Health and Science University, Portland, OR, United States.
- Division of Clinical Immunology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
- Department of Pediatrics, National Jewish Health, Denver, CO, United States.
- Department of Dermatology, University of Alabama at Birmingham, Birmingham, AL, United States.
- Department of Pediatrics, University of Alabama at Birmingham, Birmingham, AL, United States.
- Division of Dermatology, Washington University School of Medicine, St. Louis, MO, United States.
- Division of Immunology and Allergy, Sidra Medicine and Hamad Medical Corporation, Doha, Qatar.
- Center for Pediatric and Adolescent Medicine, University Medical Hospital Mainz, Mainz, Germany.
- Institute of Pathology, Faculty of Medicine, Ludwig-Maximilians-Universität München, Munich, Germany.
- Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine, Charité - Universitätsmedizin Berlin, Berlin, Germany.
- Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Berlin, Germany.
- Berlin Institute of Health at Charité - Universitätsmedizin Berlin, Berlin, Germany.
- Labor Berlin GmbH, Department of Immunology, Berlin, Germany.
- Division of Allergy Immunology, Department of Pediatrics, The Children's Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States.
Abstract
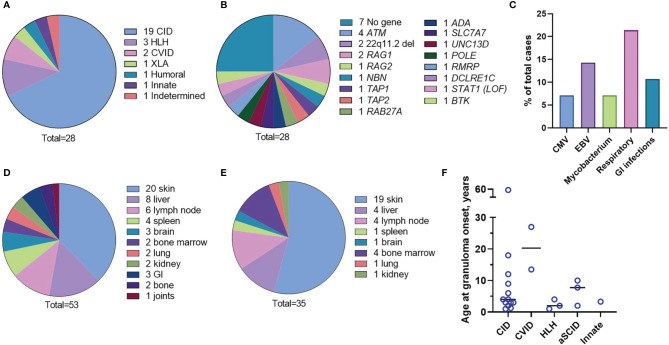
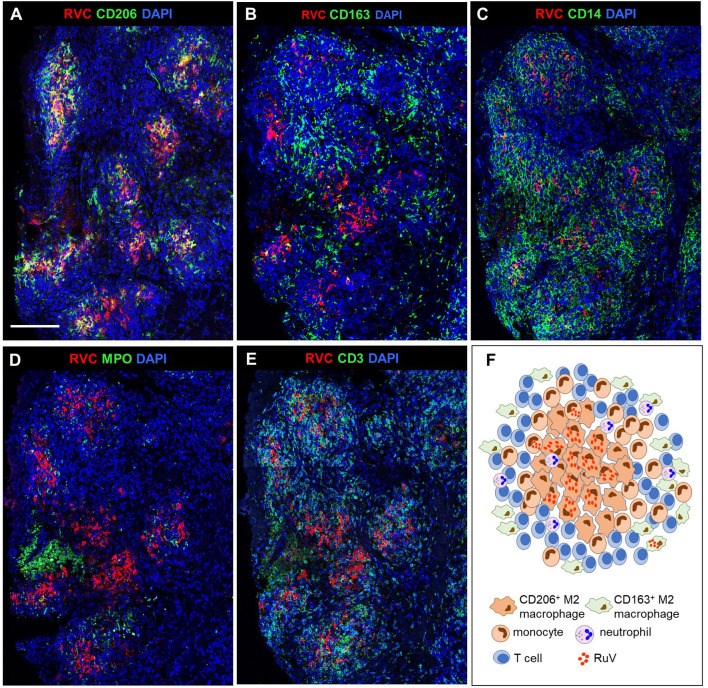
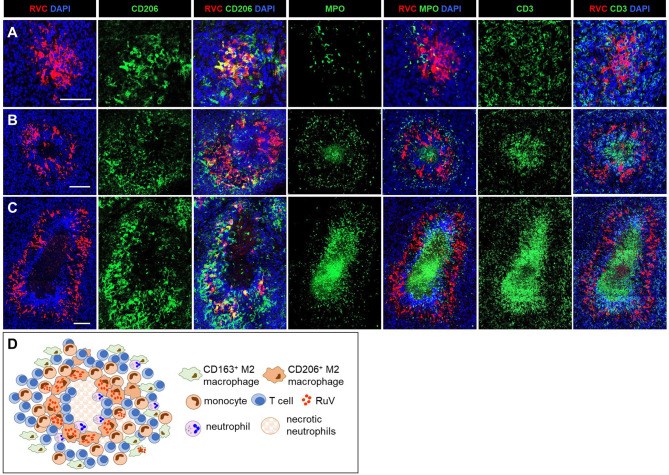
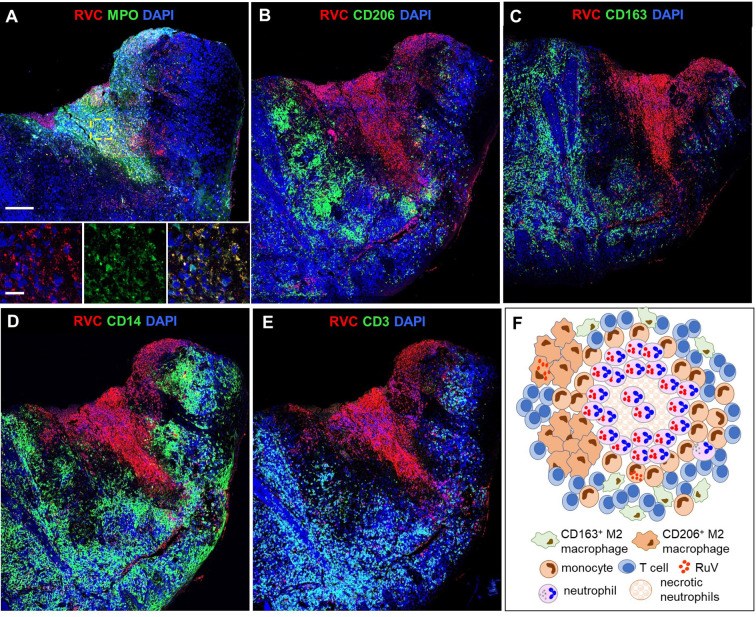
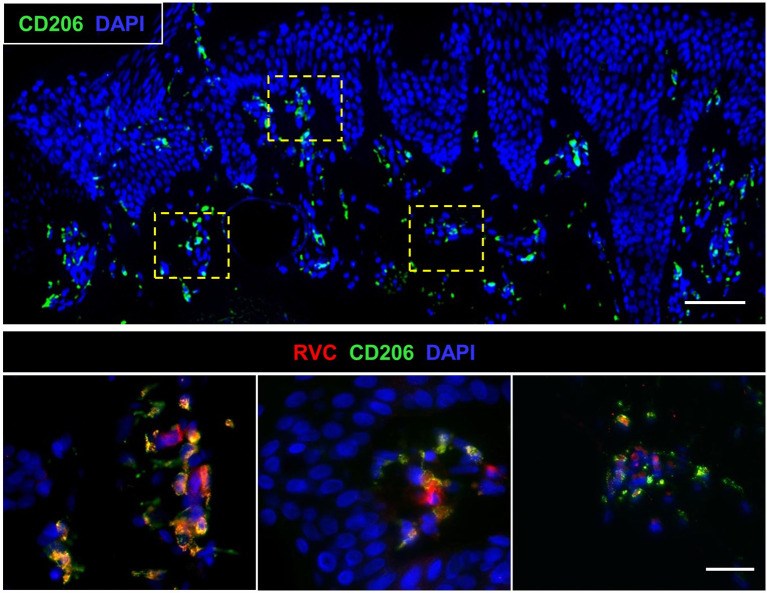
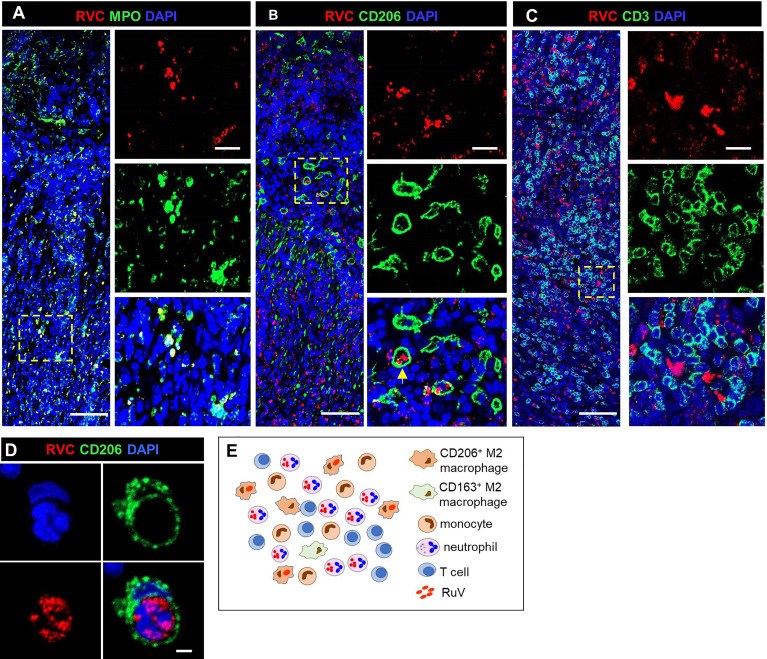
Rubella virus (RuV) has recently been found in association with granulomatous inflammation of the skin and several internal organs in patients with inborn errors of immunity (IEI). The cellular tropism and molecular mechanisms of RuV persistence and pathogenesis in select immunocompromised hosts are not clear. We provide clinical, immunological, virological, and histological data on a cohort of 28 patients with a broad spectrum of IEI and RuV-associated granulomas in skin and nine extracutaneous tissues to further delineate this relationship. Combined immunodeficiency was the most frequent diagnosis (67.8%) among patients. Patients with previously undocumented conditions, i.e., humoral immunodeficiencies, a secondary immunodeficiency, and a defect of innate immunity were identified as being susceptible to RuV-associated granulomas. Hematopoietic cell transplantation was the most successful treatment in this case series resulting in granuloma resolution; steroids, and TNF-α and IL-1R inhibitors were moderately effective. In addition to M2 macrophages, neutrophils were identified by immunohistochemical analysis as a novel cell type infected with RuV. Four patterns of RuV-associated granulomatous inflammation were classified based on the structural organization of granulomas and identity and location of cell types harboring RuV antigen. Identification of conditions that increase susceptibility to RuV-associated granulomas combined with structural characterization of the granulomas may lead to a better understanding of the pathogenesis of RuV-associated granulomas and discover new targets for therapeutic interventions.
Keywords: granuloma treatments; granulomatous inflammation; inborn errors of immunity; macrophages; neutrophils; primary immunodeficiency; skin lesion; vaccine-derived rubella viruses.
Conflict of interest statement
MA is employed by Sidra Medicine and Hamad Medical Corporation, Qatar. HB is employed by Labor Berlin GmbH, Germany. JH received grants from Immune Deficiency Foundation, the US immunodeficiency network, Chao-physician Scientist award, the Texas Medical Center Digestive Diseases Center and the Jeffrey Modell Foundation. JH received honorarium, consultation fees from Horizon, Pharming, Baxalta, CSL Behring, the National guard, and Al-Faisal University Hospital. TPA received consultation fees from Horizon, Pharming, CSL Behring. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
Rubella virus-associated chronic inflammation in primary immunodeficiency diseases.
Perelygina L, Icenogle J, Sullivan KE.Curr Opin Allergy Clin Immunol. 2020 Dec;20(6):574-581. doi: 10.1097/ACI.0000000000000694.PMID: 33044342 Free PMC article. Review.
Wanat KA, Perelygina L, Chen MH, Hao L, Abernathy E, Bender NR, Shields BE, Wilson BD, Crosby D, Routes J, Samimi SS, Haun PL, Sokumbi O, Icenogle JP, Sullivan KE, Rosenbach M, Drolet BA.JAMA Dermatol. 2022 Jun 1;158(6):626-633. doi: 10.1001/jamadermatol.2022.0828.PMID: 35338705 Free PMC article.
Cutaneous Granulomas Associated with Rubella Virus: A Clinical Review.
Zhang D, Wanat KA, Perelygina L, Rosenbach M, Haun PL, Drolet BA, Shields BE.J Am Acad Dermatol. 2023 Jun 2:S0190-9622(23)00998-2. doi: 10.1016/j.jaad.2023.05.058. Online ahead of print.PMID: 37271455 Review.
Buchbinder D, Hauck F, Albert MH, Rack A, Bakhtiar S, Shcherbina A, Deripapa E, Sullivan KE, Perelygina L, Eloit M, Neven B, Pérot P, Moshous D, Suarez F, Bodemer C, Bonilla FA, Vaz LE, Krol AL, Klein C, Seppanen M, Nugent DJ, Singh J, Ochs HD.J Clin Immunol. 2019 Jan;39(1):81-89. doi: 10.1007/s10875-018-0581-0. Epub 2019 Jan 3.PMID: 30607663 Free PMC article.
Granulomatous Dermatitis Associated With Rubella Virus Infection in an Adult With Immunodeficiency.
Shields BE, Perelygina L, Samimi S, Haun P, Leung T, Abernathy E, Chen MH, Hao L, Icenogle J, Drolet B, Wilson B, Bryer JS, England R, Blumberg E, Wanat KA, Sullivan K, Rosenbach M.JAMA Dermatol. 2021 Jul 1;157(7):842-847. doi: 10.1001/jamadermatol.2021.1577.PMID: 34037685 Free PMC article.
Cited by
Deng S, Rao S, Wang AR, Shi W.Front Genet. 2023 Mar 16;14:1115027. doi: 10.3389/fgene.2023.1115027. eCollection 2023.PMID: 37007969 Free PMC article.
Granulomatous inflammation in inborn errors of immunity.
Sacco KA, Gazzin A, Notarangelo LD, Delmonte OM.Front Pediatr. 2023 Feb 20;11:1110115. doi: 10.3389/fped.2023.1110115. eCollection 2023.PMID: 36891233 Free PMC article. Review.
Yonkof JR, Basu A, Redmond MT, Dobbs AK, Perelygina L, Notarangelo LD, Abraham RS, Rangarajan HG.Pediatr Blood Cancer. 2023 May;70(5):e30183. doi: 10.1002/pbc.30183. Epub 2022 Dec 30.PMID: 36583469 No abstract available.
Bonner KE, Sukerman E, Liko J, Lanzieri TM, Sutton M, DeBess E, Leesman C, Icenogle J, Hao L, Chen MH, Faisthalab R, Leman RF, Cieslak PR, DeRavin SS, Perelygina L.Front Immunol. 2022 Dec 8;13:1075351. doi: 10.3389/fimmu.2022.1075351. eCollection 2022.PMID: 36569925 Free PMC article.
Keeler EL, Vukmirovic M, Yan X, Gulino K, Ghedin E, Kaminski N, Sullivan KE, Bushman FD, Collman RG, Rosenbach M.Sarcoidosis Vasc Diffuse Lung Dis. 2022 Dec 19;39(4):e2022040. doi: 10.36141/svdld.v39i4.13407.PMID: 36533601 Free PMC article.
KMEL References
References
-
- Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. . Human Inborn Errors of Immunity: 2019 Update on the Classification From the International Union of Immunological Societies Expert Committee. J Clin Immunol (2020) 40(1):24–64. doi: 10.1007/s10875-019-00737-x - DOI - PMC - PubMed
-
- Leung J, Sullivan KE, Perelygina L, Icenogle JP, Fuleihan RL, Lanzieri TM. Prevalence of Granulomas in Patients With Primary Immunodeficiency Disorders, United States: Data From National Health Care Claims and the US Immunodeficiency Network Registry. J Clin Immunol (2018) 38(6):717–26. doi: 10.1007/s10875-018-0534-7 - DOI - PMC - PubMed
-
- Plotkin S, Reef S, Cooper L, Alford CA, J. R, Klein J, et al. . "Rubella," in Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA: Elsveier; (2011) p. 861–98.
-
- Rawls WE. Viral Persistence in Congenital Rubella. Prog Med Virol (1974) 18:273–88. - PubMed
-
- Bodemer C, Sauvage V, Mahlaoui N, Cheval J, Couderc T, Leclerc-Mercier S, et al. . Live Rubella Virus Vaccine Long-Term Persistence as an Antigenic Trigger of Cutaneous Granulomas in Patients With Primary Immunodeficiency. Clin Microbiol Infect (2014) 20(10):O656–663. doi: 10.1111/1469-0691.12573 - DOI - PubMed
-
- Perelygina L, Plotkin S, Russo P, Hautala T, Bonilla F, Ochs HD, et al. . Rubella Persistence in Epidermal Keratinocytes and Granuloma M2 Macrophages in Patients With Primary Immunodeficiencies. J Allergy Clin Immunol (2016) 138 1436-1439(5):e1411. doi: 10.1016/j.jaci.2016.06.030 - DOI - PMC - PubMed
-
- Buchbinder D, Hauck F, Albert MH, Rack A, Bakhtiar S, Shcherbina A, et al. . Rubella Virus-Associated Cutaneous Granulomatous Disease: A Unique Complication in Immune-Deficient Patients, Not Limited to DNA Repair Disorders. J Clin Immunol (2019) 39(1):81–9. doi: 10.1007/s10875-018-0581-0 - DOI - PMC - PubMed
-
- Perelygina L, Chen MH, Suppiah S, Adebayo A, Abernathy E, Dorsey M, et al. . Infectious Vaccine-Derived Rubella Viruses Emerge, Persist, and Evolve in Cutaneous Granulomas of Children With Primary Immunodeficiencies. PloS Pathog (2019) 15(10):e1008080. doi: 10.1371/journal.ppat.1008080 - DOI - PMC - PubMed
-
- Millar JA, Butler JR, Evans S, Mattila JT, Linderman JJ, Flynn JL, et al. . Spatial Organization and Recruitment of Non-Specific T Cells May Limit T Cell-Macrophage Interactions Within Mycobacterium Tuberculosis Granulomas. Front Immunol (2020) 11:613638. doi: 10.3389/fimmu.2020.613638 - DOI - PMC - PubMed
-
- Carneiro-Sampaio M, Moraes-Vasconcelos D, Kokron CM, Jacob CM, Toledo-Barros M, Dorna MB, et al. . Primary Immunodeficiency Diseases in Different Age Groups: A Report on 1,008 Cases From a Single Brazilian Reference Center. J Clin Immunol (2013) 33(4):716–24. doi: 10.1007/s10875-013-9865-6 - DOI - PubMed
-
- Perelygina L, Buchbinder D, Dorsey MJ, Eloit M, Hauck F, Hautala T, et al. . Outcomes for Nitazoxanide Treatment in a Case Series of Patients With Primary Immunodeficiencies and Rubella Virus-Associated Granuloma. J Clin Immunol (2019) 39(1):112–7. doi: 10.1007/s10875-019-0589-0 - DOI - PMC - PubMed