Hematopoietic stem cell transplantation outcomes for 11 patients with dedicator of cytokinesis 8 deficiency
Waleed Al-Herz 1, Julia I Chu 2, Jet van der Spek 3, Raj Raghupathy 4, Michel J Massaad 5, Sevgi Keles 5, Catherine M Biggs 5, Lucinda Cockerton 6, Janet Chou 5, Ghassan Dbaibo 7, Scott A Elisofon 8, Rima Hanna-Wakim 7, Heung Bae Kim 9, Leslie E Lehmann 2, Douglas R McDonald 5, Luigi D Notarangelo 5, Paul Veys 6, Talal A Chatila 5, Raif S Geha 5, H Bobby Gaspar 10, Sung-Yun Pai 11
Affiliations
Affiliations
- Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait; Department of Pediatrics, Al-Sabah Hospital, Kuwait City, Kuwait.
- Division of Hematology-Oncology, Boston Children's Hospital, Boston, Mass; Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston, Mass.
- Division of Hematology-Oncology, Boston Children's Hospital, Boston, Mass.
- Department of Microbiology, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait.
- Division of Immunology, Boston Children's Hospital, Boston, Mass.
- Great Ormond Street Hospital for Children, NHS Foundation Trust, London, United Kingdom.
- Division of Pediatric Infectious Diseases, American University of Beirut, Beirut, Lebanon.
- Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, Mass.
- Department of Surgery, Boston Children's Hospital, Boston, Mass.
- Great Ormond Street Hospital for Children, NHS Foundation Trust, London, United Kingdom; UCL Institute of Child Health, London, United Kingdom.
- Division of Hematology-Oncology, Boston Children's Hospital, Boston, Mass; Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston, Mass. Electronic address: sung-yun.pai@childrens.harvard.edu.
Abstract
Background: Dedicator of cytokinesis 8 (DOCK8) deficiency can be cured by allogeneic hematopoietic stem cell transplantation (HSCT). Reports of outcomes are still limited.
Objective: We sought to analyze the results of HSCT in patients with DOCK8 deficiency and report whether approaches resulting in mixed chimerism result in clinically relevant immune reconstitution.
Methods: We performed a retrospective chart review of 11 patients with DOCK8 deficiency and measured DOCK8 expression and cytokine production.
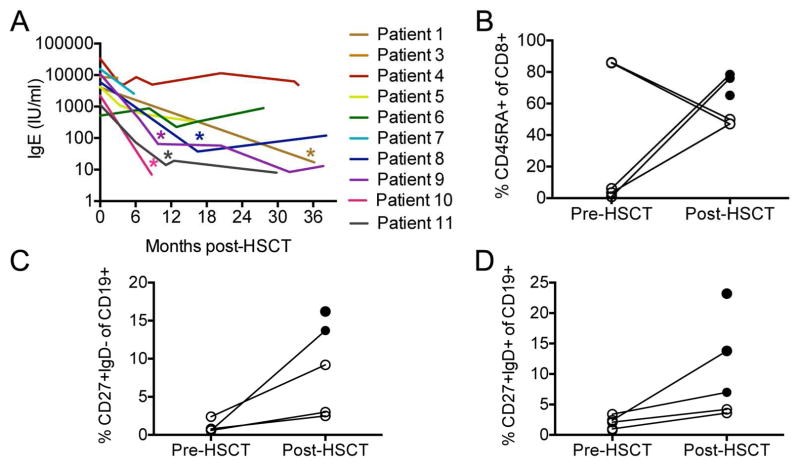
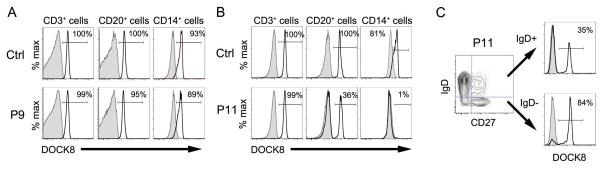
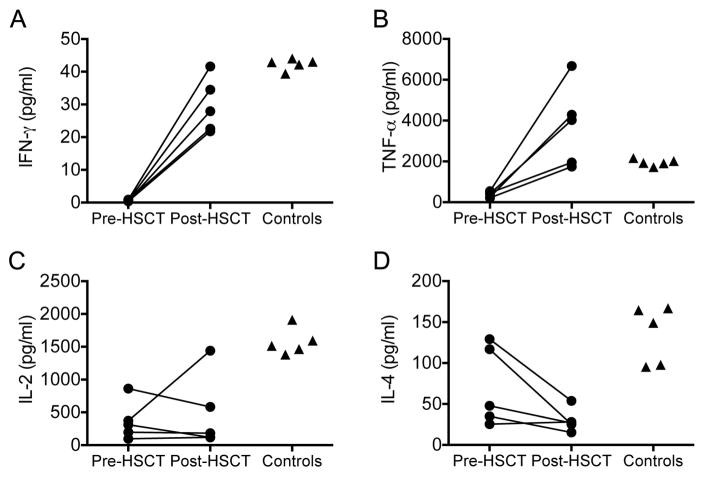
Results: Of 11 patients, 7 received HSCT from related and 4 from unrelated donors; 9 patients received busulfan-based conditioning regimens. Survival was excellent (10 [91%] of 11 patients alive), including a patient who had undergone liver transplantation. Patients showed significant improvements in the frequency and severity of infections. Although eczema resolved in all, food allergies and high IgE levels persisted in some patients. Lymphopenia, eosinophilia, low numbers of naive CD8(+) T cells and switched memory B cells, and TH1/TH2 cytokine imbalance improved in most patients. Although the 8 matched related or unrelated donor recipients had full donor chimerism, all 3 recipients of mismatched unrelated donor HSCT had high levels of donor T-cell chimerism and low B-cell and myeloid cell chimerism (0% to 46%). Almost all switched memory B cells were of donor origin. All patients, including those with mixed chimerism, mounted robust antibody responses to vaccination.
Conclusion: Allogeneic HSCT ameliorated the infectious and atopic symptoms of patients with DOCK8 deficiency. In patients with mixed chimerism, selective advantage for donor-derived T cells and switched memory B cells promoted restoration of cellular and humoral immunity and protection against opportunistic infection.
Keywords: Primary immunodeficiency; conditioning; dedicator of cytokinesis 8 deficiency; hematopoietic stem cell transplantation; mixed chimerism.
Figures
Similar articles
Raedler J, Magg T, Rohlfs M, Klein C, Vallée T, Hauck F, Albert MH.J Clin Immunol. 2021 Oct;41(7):1536-1548. doi: 10.1007/s10875-021-01069-5. Epub 2021 Jun 2.PMID: 34080085 Free PMC article.
Boztug H, Karitnig-Weiß C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, Belohradsky BH, Mann G, Horcher E, Rümmele-Waibel A, Geyeregger R, Lakatos K, Peters C, Lawitschka A, Matthes-Martin S.Pediatr Hematol Oncol. 2012 Oct;29(7):585-94. doi: 10.3109/08880018.2012.714844. Epub 2012 Aug 16.PMID: 22897717
Hematopoietic Stem Cell Transplantation as Treatment for Patients with DOCK8 Deficiency.
Aydin SE, Freeman AF, Al-Herz W, Al-Mousa HA, Arnaout RK, Aydin RC, Barlogis V, Belohradsky BH, Bonfim C, Bredius RG, Chu JI, Ciocarlie OC, Doğu F, Gaspar HB, Geha RS, Gennery AR, Hauck F, Hawwari A, Hickstein DD, Hoenig M, Ikinciogullari A, Klein C, Kumar A, Ifversen MRS, Matthes S, Metin A, Neven B, Pai SY, Parikh SH, Picard C, Renner ED, Sanal Ö, Schulz AS, Schuster F, Shah NN, Shereck EB, Slatter MA, Su HC, van Montfrans J, Woessmann W, Ziegler JB, Albert MH; Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and the European Society for Primary Immunodeficiencies.J Allergy Clin Immunol Pract. 2019 Mar;7(3):848-855. doi: 10.1016/j.jaip.2018.10.035. Epub 2018 Nov 2.PMID: 30391550 Free PMC article.
DOCK8 deficiency: Insights into pathophysiology, clinical features and management.
Biggs CM, Keles S, Chatila TA.Clin Immunol. 2017 Aug;181:75-82. doi: 10.1016/j.clim.2017.06.003. Epub 2017 Jun 15.PMID: 28625885 Free PMC article. Review.
Current Status of Dedicator of Cytokinesis-Associated Immunodeficiency: DOCK8 and DOCK2.
Dimitrova D, Freeman AF.Dermatol Clin. 2017 Jan;35(1):11-19. doi: 10.1016/j.det.2016.07.002.PMID: 27890234 Free PMC article. Review.
Cited by
Kono A, Wakamatsu M, Umezawa Y, Muramatsu H, Fujiwara H, Tomomasa D, Inoue K, Hattori K, Mitsui T, Takada H, Minegishi Y, Takahashi Y, Yamamoto M, Mori T, Kanegane H.Int J Hematol. 2023 May 3. doi: 10.1007/s12185-023-03613-y. Online ahead of print.PMID: 37131080
El-Sayed ZA, El-Ghoneimy DH, Ortega-Martell JA, Radwan N, Aldave JC, Al-Herz W, Al-Nesf MA, Condino-Neto A, Cole T, Eley B, Erwa NHH, Espinosa-Padilla S, Faria E, Rosario Filho NA, Fuleihan R, Galal N, Garabedian E, Hintermeyer M, Imai K, Irani C, Kamal E, Kechout N, Klocperk A, Levin M, Milota T, Ouederni M, Paganelli R, Pignata C, Qamar FN, Quinti I, Qureshi S, Radhakrishnan N, Rezaei N, Routes J, Singh S, Siniah S, Abdel-Hakam Taha I, Tanno LK, Van Dort B, Volokha A, Sullivan K.World Allergy Organ J. 2022 Jun 17;15(6):100657. doi: 10.1016/j.waojou.2022.100657. eCollection 2022 Jun.PMID: 35783543 Free PMC article.
CRISPR/Cas-Based Gene Editing Strategies for DOCK8 Immunodeficiency Syndrome.
Ravendran S, Hernández SS, König S, Bak RO.Front Genome Ed. 2022 Mar 17;4:793010. doi: 10.3389/fgeed.2022.793010. eCollection 2022.PMID: 35373187 Free PMC article.
Holland EM, Gonzalez C, Levy E, Valera VA, Chalfin H, Klicka-Skeels J, Yates B, Kleiner DE, Hadigan C, Dave H, Shalabi H, Hickstein DD, Su HC, Grimley M, Freeman AF, Shah NN.Front Immunol. 2021 Dec 17;12:801281. doi: 10.3389/fimmu.2021.801281. eCollection 2021.PMID: 34975916 Free PMC article.