Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury
Affiliations
Affiliations
- Department of Pharmacology and Therapeutics, Faculty of Pharmacy, Kuwait University, Safat 13110, Kuwait. Electronic address: ahmet.oz@ku.edu.kw.
- Department of Anatomy and Cellular Biology, Khalifa University, Abu Dhabi, United Arab Emirates; Center for Biotechnology, Khalifa University of Science and Technology, Abu Dhabi, United Arab Emirates.
Abstract
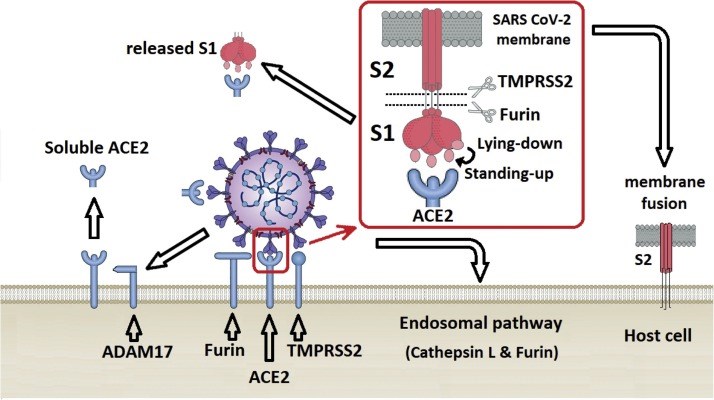
The recent emergence of coronavirus disease-2019 (COVID-19) as a pandemic affecting millions of individuals has raised great concern throughout the world, and the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was identified as the causative agent for COVID-19. The multifunctional protein angiotensin converting enzyme 2 (ACE2) is accepted as its primary target for entry into host cells. In its enzymatic function, ACE2, like its homologue ACE, regulates the renin-angiotensin system (RAS) critical for cardiovascular and renal homeostasis in mammals. Unlike ACE, however, ACE2 drives an alternative RAS pathway by degrading Ang-II and thus operates to balance RAS homeostasis in the context of hypertension, heart failure, and cardiovascular as well as renal complications of diabetes. Outside the RAS, ACE2 hydrolyzes key peptides, such as amyloid-β, apelin, and [des-Arg9]-bradykinin. In addition to its enzymatic functions, ACE2 is found to regulate intestinal amino acid homeostasis and the gut microbiome. Although the non-enzymatic function of ACE2 as the entry receptor for SARS-CoV-2 has been well established, the contribution of enzymatic functions of ACE2 to the pathogenesis of COVID-19-related lung injury has been a matter of debate. A complete understanding of this central enzyme may begin to explain the various symptoms and pathologies seen in SARS-CoV-2 infected individuals, and may aid in the development of novel treatments for COVID-19.
Keywords: ACE2; ARDS; Angiotensin II; COVID-19; Coronavirus; SARS.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that there are no conflicts of interest.
Figures
Similar articles
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.
Kuba K, Yamaguchi T, Penninger JM.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021.PMID: 35003058 Free PMC article. Review.
Aleksova A, Gagno G, Sinagra G, Beltrami AP, Janjusevic M, Ippolito G, Zumla A, Fluca AL, Ferro F.Int J Mol Sci. 2021 Apr 26;22(9):4526. doi: 10.3390/ijms22094526.PMID: 33926110 Free PMC article. Review.
Cheng H, Wang Y, Wang GQ.J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5.PMID: 32221983 Free PMC article. Review.
Datta PK, Liu F, Fischer T, Rappaport J, Qin X.Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020.PMID: 32642005 Free PMC article. Review.
Angiotensin-converting enzyme 2 and COVID-19 in cardiorenal diseases.
Sharma RK, Li J, Krishnan S, Richards EM, Raizada MK, Mohandas R.Clin Sci (Lond). 2021 Jan 15;135(1):1-17. doi: 10.1042/CS20200482.PMID: 33399851 Free PMC article. Review.
Cited by
Adipokines as Diagnostic and Prognostic Markers for the Severity of COVID-19.
Grewal T, Buechler C.Biomedicines. 2023 Apr 27;11(5):1302. doi: 10.3390/biomedicines11051302.PMID: 37238973 Free PMC article. Review.
Atwah B, Iqbal MS, Kabrah S, Kabrah A, Alghamdi S, Tabassum A, Baghdadi MA, Alzahrani H.Vaccines (Basel). 2023 Mar 1;11(3):561. doi: 10.3390/vaccines11030561.PMID: 36992148 Free PMC article.
SARS-CoV-2 infection and its effects on the endocrine system.
Steenblock C, Toepfner N, Beuschlein F, Perakakis N, Mohan Anjana R, Mohan V, Mahapatra NR, Bornstein SR.Best Pract Res Clin Endocrinol Metab. 2023 Mar 5:101761. doi: 10.1016/j.beem.2023.101761. Online ahead of print.PMID: 36907787 Free PMC article. Review.
Nejat R, Torshizi MF, Najafi DJ.Vaccines (Basel). 2023 Jan 17;11(2):204. doi: 10.3390/vaccines11020204.PMID: 36851081 Free PMC article. Review.
Bellavite P, Ferraresi A, Isidoro C.Biomedicines. 2023 Feb 3;11(2):451. doi: 10.3390/biomedicines11020451.PMID: 36830987 Free PMC article. Review.
KMEL References
References
-
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):E1–9. doi: 10.1161/01.res.87.5.e1. - DOI - PubMed
-
- Turner A.J. ACE2 cell biology, regulation, and physiological functions. In: Unger T., Steckelings U.M., dos Santos R.A.S., editors. The Protective Arm of the Renin Angiotensin System: Functional Aspects and Therapeutic Implications. Elsevier Inc.; Amsterdam: 2015. pp. 185–189.
-
- Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277(17):14838–14843. doi: 10.1074/jbc.M200581200. - DOI - PubMed
-
- Iwata M., Greenberg B.H. Ectodomain shedding of ACE and ACE2 as regulators of their protein functions. Curr. Enzym. Inhib. 2011;7(1):42–55. doi: 10.2174/157340811795713756. - DOI
-
- Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S., Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 2008;52(9):750–754. doi: 10.1016/j.jacc.2008.02.088. - DOI - PMC - PubMed
-
- Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O., Poglitsch M., Lazartigues E. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ. Res. 2017;121(1):43–55. doi: 10.1161/CIRCRESAHA.116.310509. - DOI - PMC - PubMed
-
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. - DOI - PMC - PubMed
-
- Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. - DOI - PubMed
-
- Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K.A., Gerhart M., Davis R., Fitzner J.N., Johnson R.S., Paxton R.J., March C.J., Cerretti D.P. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385(6618):729–733. doi: 10.1038/385729a0. - DOI - PubMed
-
- Moss M.L., Jin S.L., Milla M.E., Bickett D.M., Burkhart W., Carter H.L., Chen W.J., Clay W.C., Didsbury J.R., Hassler D., Hoffman C.R., Kost T.A., Lambert M.H., Leesnitzer M.A., McCauley P., McGeehan G., Mitchell J., Moyer M., Pahel G., Rocque W., Overton L.K., Schoenen F., Seaton T., Su J.L., Becherer J.D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385(6618):733–736. doi: 10.1038/385733a0. - DOI - PubMed
-
- Scott A.J., O’Dea K.P., O’Callaghan D., Williams L., Dokpesi J.O., Tatton L., Handy J.M., Hogg P.J., Takata M. Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor α-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J. Biol. Chem. 2011;286(41):35466–35476. doi: 10.1074/jbc.M111.277434. - DOI - PMC - PubMed
-
- Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297(1):L84–96. doi: 10.1152/ajplung.00071.2009. - DOI - PMC - PubMed
-
- Arendse L.B., Danser A.H.J., Poglitsch M., Touyz R.M., Burnett J.C., Llorens-Cortes C., Ehlers M.R., Sturrock E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019;71(4):539–570. doi: 10.1124/pr.118.017129. - DOI - PMC - PubMed
-
- Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. - DOI - PMC - PubMed
-
- Shao M., Wen Z.-B., Yang H.-H., Zhang C.-Y., Xiong J.-B., Guan X.-X., Zhong W.-J., Jiang H.-L., Sun C.-C., Luo X.-Q., He X.-F., Zhou Y., Guan C.-X. Exogenous angiotensin (1-7) directly inhibits epithelial-mesenchymal transformation induced by transforming growth factor-β1 in alveolar epithelial cells. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109193. - DOI - PubMed
-
- Hao P.P., Yang J.M., Zhang M.X., Zhang K., Chen Y.G., Zhang C., Zhang Y. Angiotensin-(1-7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015;308(9):H1007–1019. doi: 10.1152/ajpheart.00563.2014. - DOI - PubMed
-
- Xue H., Zhou L., Yuan P., Wang Z., Ni J., Yao T., Wang J., Huang Y., Yu C., Lu L. Counteraction between angiotensin II and angiotensin-(1-7) via activating angiotensin type I and Mas receptor on rat renal mesangial cells. Regul. Pept. 2012;177(1–3):12–20. doi: 10.1016/j.regpep.2012.04.002. - DOI - PubMed
-
- Zhai C.-g., Xu Y.-y., Tie Y.-y., Zhang Y., Chen W.-q., Ji X.-p., Mao Y., Qiao L., Cheng J., Xu Q.-b., Zhang C. DKK3 overexpression attenuates cardiac hypertrophy and fibrosis in an angiotensin-perfused animal model by regulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways. J. Mol. Cell. Cardiol. 2018;114:243–252. doi: 10.1016/j.yjmcc.2017.11.018. - DOI - PubMed
-
- Read C., Nyimanu D., Williams T.L., Huggins D.J., Sulentic P., Macrae R.G.C., Yang P., Glen R.C., Maguire J.J., Davenport A.P. International union of basic and clinical pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that Elabela/Toddler is a second endogenous peptide ligand. Pharmacol. Rev. 2019;71(4):467–502. doi: 10.1124/pr.119.017533. - DOI - PMC - PubMed
-
- Wang W., McKinnie S.M., Farhan M., Paul M., McDonald T., McLean B., Llorens-Cortes C., Hazra S., Murray A.G., Vederas J.C., Oudit G.Y. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17: physiological effects in the cardiovascular system. Hypertension. 2016;68(2):365–377. doi: 10.1161/hypertensionaha.115.06892. - DOI - PubMed
-
- Zhang Z.Z., Wang W., Jin H.Y., Chen X., Cheng Y.W., Xu Y.L., Song B., Penninger J.M., Oudit G.Y., Zhong J.C. Apelin is a negative regulator of angiotensin II-Mediated adverse myocardial remodeling and dysfunction. Hypertension. 2017;70(6):1165–1175. doi: 10.1161/hypertensionaha.117.10156. - DOI - PubMed
-
- Sato T., Sato C., Kadowaki A., Watanabe H., Ho L., Ishida J., Yamaguchi T., Kimura A., Fukamizu A., Penninger J.M., Reversade B., Ito H., Imai Y., Kuba K. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc. Res. 2017;113(7):760–769. doi: 10.1093/cvr/cvx061. - DOI - PubMed
-
- Sabry M.M., Mahmoud M.M., Shoukry H.S., Rashed L., Kamar S.S., Ahmed M.M. Interactive effects of apelin, renin-angiotensin system and nitric oxide in treatment of obesity-induced type 2 diabetes mellitus in male albino rats. Arch. Physiol. Biochem. 2019;125(3):244–254. doi: 10.1080/13813455.2018.1453521. - DOI - PubMed
-
- Masoud A.G., Lin J., Azad A.K., Farhan M.A., Fischer C., Zhu L.F., Zhang H., Sis B., Kassiri Z., Moore R.B., Kim D., Anderson C.C., Vederas J.C., Adam B.A., Oudit G.Y., Murray A.G. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Invest. 2020;130(1):94–107. doi: 10.1172/jci128469. - DOI - PMC - PubMed
-
- Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Jr., Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314(1):L17–l31. doi: 10.1152/ajplung.00498.2016. - DOI - PMC - PubMed
-
- More A.S., Kim H.M., Khang G., Hildebrandt T., Bernlöhr C., Doods H., Vanhoutte P.M., Wu D. Des-Arg9-bradykinin causes kinin B1 receptor mediated endothelium-independent contractions in endotoxin-treated porcine coronary arteries. Pharmacol. Res. 2014;90:18–24. doi: 10.1016/j.phrs.2014.09.001. - DOI - PubMed
-
- Ehrenfeld P., Conejeros I., Pavicic M.F., Matus C.E., Gonzalez C.B., Quest A.F.G., Bhoola K.D., Poblete M.T., Burgos R.A., Figueroa C.D. Activation of kinin B1 receptor increases the release of metalloproteases-2 and -9 from both estrogen-sensitive and -insensitive breast cancer cells. Cancer Lett. 2011;301(1):106–118. doi: 10.1016/j.canlet.2010.09.020. - DOI - PubMed
-
- Matus C.E., Ehrenfeld P., Pavicic F., González C.B., Concha M., Bhoola K.D., Burgos R.A., Figueroa C.D. Activation of the human keratinocyte B1 bradykinin receptor induces expression and secretion of metalloproteases 2 and 9 by transactivation of epidermal growth factor receptor. Exp. Dermatol. 2016;25(9):694–700. doi: 10.1111/exd.13038. - DOI - PubMed
-
- Dey M., Baldys A., Sumter D.B., Göoz P., Luttrell L.M., Raymond J.R., Göoz M. Bradykinin decreases podocyte permeability through ADAM17-dependent epidermal growth factor receptor activation and zonula occludens-1 rearrangement. J. Pharmacol. Exp. Ther. 2010;334(3):775–783. doi: 10.1124/jpet.110.168054. - DOI - PMC - PubMed
-
- Lorke D.E., Petroianu G., Oz M. α7-nicotinic acetylcholine receptors and β-amyloid peptides in Alzheimer’s disease. In: Li M.D., editor. Nicotinic Acetylcholine Receptor Technologies. Springer Science + Business Media; Berlin, Heidelberg: 2016. pp. 171–205.
-
- Zou K., Yamaguchi H., Akatsu H., Sakamoto T., Ko M., Mizoguchi K., Gong J.S., Yu W., Yamamoto T., Kosaka K., Yanagisawa K., Michikawa M. Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J. Neurosci. 2007;27(32):8628–8635. doi: 10.1523/jneurosci.1549-07.2007. - DOI - PMC - PubMed
-
- Tikhonova M.A., Amstislavskaya T.G., Belichenko V.M., Fedoseeva L.A., Kovalenko S.P., Pisareva E.E., Avdeeva A.S., Kolosova N.G., Belyaev N.D., Aftanas L.I. Modulation of the expression of genes related to the system of amyloid-beta metabolism in the brain as a novel mechanism of ceftriaxone neuroprotective properties. BMC Neurosci. 2018;19(Suppl 1):13. doi: 10.1186/s12868-018-0412-5. - DOI - PMC - PubMed
-
- Wang X.L., Iwanami J., Min L.J., Tsukuda K., Nakaoka H., Bai H.Y., Shan B.S., Kan-No H., Kukida M., Chisaka T., Yamauchi T., Higaki A., Mogi M., Horiuchi M. Deficiency of angiotensin-converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech. Dis. 2016;2:16024. doi: 10.1038/npjamd.2016.24. - DOI - PMC - PubMed
-
- Evans C.E., Miners J.S., Piva G., Willis C.L., Heard D.M., Kidd E.J., Good M.A., Kehoe P.G. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139(3):485–502. doi: 10.1007/s00401-019-02098-6. - DOI - PMC - PubMed
-
- Kamel A.S., Abdelkader N.F., Abd El-Rahman S.S., Emara M., Zaki H.F., Khattab M.M. Stimulation of ACE2/ANG(1-7)/mas axis by diminazene ameliorates Alzheimer’s disease in the D-Galactose-Ovariectomized rat model: role of PI3K/Akt pathway. Mol. Neurobiol. 2018;55(10):8188–8202. doi: 10.1007/s12035-018-0966-3. - DOI - PubMed
-
- Duan R., Xue X., Zhang Q.Q., Wang S.Y., Gong P.Y., Jiang Y.E.T., Zhang Y.D. ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging (Albany NY) 2020;12(14):14819–14829. doi: 10.18632/aging.103544. - DOI - PMC - PubMed
-
- Chen J.L., Zhang D.L., Sun Y., Zhao Y.X., Zhao K.X., Pu D., Xiao Q. Angiotensin-(1-7) administration attenuates Alzheimer’s disease-like neuropathology in rats with streptozotocin-induced diabetes via Mas receptor activation. Neuroscience. 2017;346:267–277. doi: 10.1016/j.neuroscience.2017.01.027. - DOI - PubMed
-
- Uekawa K., Hasegawa Y., Senju S., Nakagata N., Ma M., Nakagawa T., Koibuchi N., Kim-Mitsuyama S. Intracerebroventricular infusion of angiotensin-(1-7) ameliorates cognitive impairment and memory dysfunction in a mouse model of alzheimer’s disease. J. Alzheimers Dis. 2016;53(1):127–133. doi: 10.3233/jad-150642. - DOI - PubMed
-
- Jiang T., Zhang Y.D., Zhou J.S., Zhu X.C., Tian Y.Y., Zhao H.D., Lu H., Gao Q., Tan L., Yu J.T. Angiotensin-(1-7) is reduced and inversely correlates with tau hyperphosphorylation in animal models of Alzheimer’s disease. Mol. Neurobiol. 2016;53(4):2489–2497. doi: 10.1007/s12035-015-9260-9. - DOI - PubMed
-
- Hay M., Polt R., Heien M.L., Vanderah T.W., Largent-Milnes T.M., Rodgers K., Falk T., Bartlett M.J., Doyle K.P., Konhilas J.P. A novel angiotensin-(1-7) glycosylated mas receptor agonist for treating vascular cognitive impairment and inflammation-related memory dysfunction. J. Pharmacol. Exp. Ther. 2019;369(1):9–25. doi: 10.1124/jpet.118.254854. - DOI - PMC - PubMed
-
- Ding Q., Shults N.V., Harris B.T., Suzuki Y.J. Angiotensin-converting enzyme 2 (ACE2) is upregulated in Alzheimer’s disease brain. bioRxiv. 2020 doi: 10.1101/2020.10.08.331157. 2020.10.08.331157. - DOI
-
- Yamazato M., Ferreira A.J., Yamazato Y., Diez-Freire C., Yuan L., Gillies R., Raizada M.K. Gene transfer of angiotensin-converting enzyme 2 in the nucleus tractus solitarius improves baroreceptor heart rate reflex in spontaneously hypertensive rats. J. Renin. Syst. 2011;12(4):456–461. doi: 10.1177/1470320311412809. - DOI - PMC - PubMed
-
- Danilczyk U., Sarao R., Remy C., Benabbas C., Stange G., Richter A., Arya S., Pospisilik J.A., Singer D., Camargo S.M., Makrides V., Ramadan T., Verrey F., Wagner C.A., Penninger J.M. Essential role for collectrin in renal amino acid transport. Nature. 2006;444(7122):1088–1091. doi: 10.1038/nature05475. - DOI - PubMed
-
- Zhang H., Wada J., Hida K., Tsuchiyama Y., Hiragushi K., Shikata K., Wang H., Lin S., Kanwar Y.S., Makino H. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J. Biol. Chem. 2001;276(20):17132–17139. doi: 10.1074/jbc.M006723200. - DOI - PubMed
-
- Malakauskas S.M., Quan H., Fields T.A., McCall S.J., Yu M.J., Kourany W.M., Frey C.W., Le T.H. Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am. J. Physiol. Renal Physiol. 2007;292(2):F533–544. doi: 10.1152/ajprenal.00325.2006. - DOI - PubMed
-
- Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A., Kuba K., Danilczyk U., Skovby F., Kleta R., Penninger J.M., Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136(3):872–882. doi: 10.1053/j.gastro.2008.10.055. - DOI - PMC - PubMed
-
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M.R., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. - DOI - PMC - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. - DOI - PMC - PubMed
-
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. - DOI - PMC - PubMed
-
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Andres C., Garcia-Cehic D., Gregori J., Piñana M., Rodriguez-Frias F., Guerrero-Murillo M., Esperalba J., Rando A., Goterris L., Codina M.G., Quer S., Martín M.C., Campins M., Ferrer R., Almirante B., Esteban J.I., Pumarola T., Antón A., Quer J. Naturally occurring SARS-CoV-2 gene deletions close to the spike S1/S2 cleavage site in the viral quasispecies of COVID19 patients. Emerg. Microbes Infect. 2020:1–48. doi: 10.1080/22221751.2020.1806735. - DOI - PubMed
-
- Jin X., Xu K., Jiang P., Lian J., Hao S., Yao H., Jia H., Zhang Y., Zheng L., Zheng N., Chen D., Yao J., Hu J., Gao J., Wen L., Shen J., Ren Y., Yu G., Wang X., Lu Y., Yu X., Yu L., Xiang D., Wu N., Lu X., Cheng L., Liu F., Wu H., Jin C., Yang X., Qian P., Qiu Y., Sheng J., Liang T., Li L., Yang Y. Virus strain from a mild COVID-19 patient in Hangzhou represents a new trend in SARS-CoV-2 evolution potentially related to Furin cleavage site. Emerg. Microbes Infect. 2020;9(1):1474–1488. doi: 10.1080/22221751.2020.1781551. - DOI - PMC - PubMed
-
- MacGowan S.A., Barton G.J. Missense variants in ACE2 are predicted to encourage and inhibit interaction with SARS-CoV-2 Spike and contribute to genetic risk in COVID-19. bioRxiv. 2020 doi: 10.1101/2020.05.03.074781. 2020.05.03.074781. - DOI
-
- Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. - DOI - PMC - PubMed
-
- Schneider M., Ackermann K., Stuart M., Wex C., Protzer U., Schätzl H.M., Gilch S. Severe acute respiratory syndrome coronavirus replication is severely impaired by MG132 due to proteasome-independent inhibition of M-calpain. J. Virol. 2012;86(18):10112–10122. doi: 10.1128/jvi.01001-12. - DOI - PMC - PubMed
-
- Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85(9):4122–4134. doi: 10.1128/jvi.02232-10. - DOI - PMC - PubMed
-
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. - DOI - PMC - PubMed
-
- Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86(12):6537–6545. doi: 10.1128/jvi.00094-12. - DOI - PMC - PubMed
-
- Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D., Morrissey C., Corey E., Montgomery B., Mostaghel E., Clegg N., Coleman I., Brown C.M., Schneider E.L., Craik C., Simon J.A., Bedalov A., Nelson P.S. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.Cd-13-1010. - DOI - PMC - PubMed
-
- Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):e105114. doi: 10.15252/embj.20105114. - DOI - PMC - PubMed
-
- Komatsu T., Suzuki Y., Imai J., Sugano S., Hida M., Tanigami A., Muroi S., Yamada Y., Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2) DNA Seq. 2002;13(4):217–220. doi: 10.1080/1042517021000021608. - DOI - PubMed
-
- Lin B., Ferguson C., White J.T., Wang S., Vessella R., True L.D., Hood L., Nelson P.S. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59(17):4180–4184. - PubMed
-
- Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779–782. doi: 10.1158/2159-8290.Cd-20-0451. - DOI - PMC - PubMed
-
- Shiryaev S.A., Remacle A.G., Ratnikov B.I., Nelson N.A., Savinov A.Y., Wei G., Bottini M., Rega M.F., Parent A., Desjardins R., Fugere M., Day R., Sabet M., Pellecchia M., Liddington R.C., Smith J.W., Mustelin T., Guiney D.G., Lebl M., Strongin A.Y. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J. Biol. Chem. 2007;282(29):20847–20853. doi: 10.1074/jbc.M703847200. - DOI - PubMed
-
- Cheng Y.-W., Chao T.-L., Li C.-L., Chiu M.-F., Kao H.-C., Wang S.-H., Pang Y.-H., Lin C.-H., Tsai Y.-M., Lee W.-H., Tao M.-H., Ho T.-C., Wu P.-Y., Jang L.-T., Chen P.-J., Chang S.-Y., Yeh S.-H. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Available at SSRN. Cell Rep. 2020 doi: 10.2139/ssrn.3613035. under review. - DOI - PMC - PubMed
-
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81(16):8722–8729. doi: 10.1128/jvi.00253-07. - DOI - PMC - PubMed
-
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. - DOI - PMC - PubMed
-
- Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88(2):1293–1307. doi: 10.1128/jvi.02202-13. - DOI - PMC - PubMed
-
- Hoffmann M., Hofmann-Winkler H., Pöhlmann S. Activation of Viruses by Host Proteases. 2018. Priming time: how cellular proteases arm coronavirus spike proteins; pp. 71–98. - DOI
-
- Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pohlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319(4):1216–1221. doi: 10.1016/j.bbrc.2004.05.114. - DOI - PMC - PubMed
-
- Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U.S.A. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. - DOI - PMC - PubMed
-
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. - DOI - PMC - PubMed
-
- Algaissi A., Agrawal A.S., Han S., Peng B.H., Luo C., Li F., Chan T.S., Couch R.B., Tseng C.K. Elevated human dipeptidyl peptidase 4 expression reduces the susceptibility of hDPP4 transgenic mice to middle east respiratory syndrome coronavirus infection and disease. J. Infect. Dis. 2019;219(5):829–835. doi: 10.1093/infdis/jiy574. - DOI - PMC - PubMed
-
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. - DOI - PMC - PubMed
-
- Winkler E.S., Bailey A.L., Kafai N.M., Nair S., McCune B.T., Yu J., Fox J.M., Chen R.E., Earnest J.T., Keeler S.P., Ritter J.H., Kang L.-I., Dort S., Robichaud A., Head R., Holtzman M.J., Diamond M.S. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21(11):1327–1335. doi: 10.1038/s41590-020-0778-2. - DOI - PMC - PubMed
-
- Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., Shadel G.S., Hepokoski M., Lei T., Wang H., Zhang J., Yuan J.X.-J., Malhotra A., Manor U., Wang S., Yuan Z.-Y., Shyy J.Y.-J. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. bioRxiv. 2020 doi: 10.1101/2020.12.04.409144. 2020.12.04.409144. - DOI - PMC - PubMed
-
- Gorshkov K., Susumu K., Chen J., Xu M., Pradhan M., Zhu W., Hu X., Breger J.C., Wolak M., Oh E. Quantum dot-conjugated SARS-CoV-2 spike pseudo-virions enable tracking of angiotensin converting enzyme 2 binding and endocytosis. ACS Nano. 2020;14(9):12234–12247. doi: 10.1021/acsnano.0c05975. - DOI - PMC - PubMed
-
- Chen I.-Y., Chang S.C., Wu H.-Y., Yu T.-C., Wei W.-C., Lin S., Chien C.-L., Chang M.-F. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus Spike-ACE2 signaling pathway. J. Virol. 2010;84(15):7703–7712. doi: 10.1128/jvi.02560-09. - DOI - PMC - PubMed
-
- He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J., Deng Y., Yang L., Li J., Cai J., Qiu L., Wen K., Xu X., Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. - DOI - PMC - PubMed
-
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., van den Brand J., Baric R., Katze M.G. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009;83(14):7062–7074. doi: 10.1128/jvi.00127-09. - DOI - PMC - PubMed
-
- Dijkman R., Jebbink M.F., Deijs M., Milewska A., Pyrc K., Buelow E., van der Bijl A., van der Hoek L. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J. Gen. Virol. 2012;93(Pt 9):1924–1929.. doi: 10.1099/vir.0.043919-0. - DOI - PubMed
-
- Wang D., Chai X.Q., Magnussen C.G., Zosky G.R., Shu S.H., Wei X., Hu S.S. Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulm. Pharmacol. Ther. 2019;58:101833. doi: 10.1016/j.pupt.2019.101833. - DOI - PMC - PubMed
-
- Wosten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. - DOI - PubMed
-
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112+. - PMC - PubMed
-
- Chen J., Xiao X., Chen S., Zhang C., Chen J., Yi D., Shenoy V., Raizada M.K., Zhao B., Chen Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61(3):681–689. doi: 10.1161/hypertensionaha.111.00202. - DOI - PMC - PubMed
-
- Yan D., Li G., Zhang Y., Liu Y. Angiotensin-converting enzyme 2 activation suppresses pulmonary vascular remodeling by inducing apoptosis through the Hippo signaling pathway in rats with pulmonary arterial hypertension. Clin. Exp. Hypertens. 2019;41(6):589–598. doi: 10.1080/10641963.2019.1583247. - DOI - PubMed
-
- Mathewson A.C., Bishop A., Yao Y., Kemp F., Ren J., Chen H., Xu X., Berkhout B., van der Hoek L., Jones I.M. Interaction of severe acute respiratory syndrome-coronavirus and NL63 coronavirus spike proteins with angiotensin converting enzyme-2. J. Gen. Virol. 2008;89(Pt 11):2741–2745. doi: 10.1099/vir.0.2008/003962-0. - DOI - PMC - PubMed
-
- Huang I.C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., Vasilieva N., Dermody T.S., Harrison S.C., Dormitzer P.R., Farzan M., Rottier P.J., Choe H. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281(6):3198–3203. doi: 10.1074/jbc.M508381200. - DOI - PMC - PubMed
-
- Wang Y., Wang Y., Luo W., Huang L., Xiao J., Li F., Qin S., Song X., Wu Y., Zeng Q., Jin F., Wang Y. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020;17(11):1522–1531. doi: 10.7150/ijms.46695. - DOI - PMC - PubMed
-
- Aguiar J.A., Tremblay B.J.-M., Mansfield M.J., Woody O., Lobb B., Banerjee A., Chandiramohan A., Tiessen N., Cao Q., Dvorkin-Gheva A., Revill S., Miller M.S., Carlsten C., Organ L., Joseph C., John A., Hanson P., Austin R., McManus B.M., Jenkins G., Mossman K., Ask K., Doxey A.C., Hirota J.A. Gene expression and <em>in situ</em> protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur. Respir. J. 2020:2001123. doi: 10.1183/13993003.01123-2020. - DOI - PMC - PubMed
-
- Hönzke K., Obermayer B., Mache C., Fatykhova D., Kessler M., Dökel S., Wyler E., Hoffmann K., Schulze J., Mieth M., Hellwig K., Biere B., Brunotte L., Mecate-Zambrano A., Hoppe J., Dohmen M., Hinze C., Elezkurtaj S., Tönnies M., Bauer T., Eggeling S., Tran H.-L., Schneider P., Neudecker J., Rückert J.-C., Schmidt-Ott K., Busch J., Klauschen F., Horst D., Radbruch H., Heppner F., Corman V.M., Niemeyer D., Müller M.A., Goffinet C., Beule D., Landthaler M., Ludwig S., Niedobitek G., Suttorp N., Witzenrath M., Gruber A., Drosten C., Sander L., Wolff T., Hippenstiel S., Hocke A.C. Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but strong virus-induced immune activation in alveolar macrophages. Cell. 2020;6(October) doi: 10.2139/ssrn.3687020. - DOI
-
- Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., 3rd, Kato T., Lee R.E., Yount B.L., Mascenik T.M., Chen G., Olivier K.N., Ghio A., Tse L.V., Leist S.R., Gralinski L.E., Schäfer A., Dang H., Gilmore R., Nakano S., Sun L., Fulcher M.L., Livraghi-Butrico A., Nicely N.I., Cameron M., Cameron C., Kelvin D.J., de Silva A., Margolis D.M., Markmann A., Bartelt L., Zumwalt R., Martinez F.J., Salvatore S.P., Borczuk A., Tata P.R., Sontake V., Kimple A., Jaspers I., O’Neal W.K., Randell S.H., Boucher R.C., Baric R.S. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–446. doi: 10.1016/j.cell.2020.05.042. e14. - DOI - PMC - PubMed
-
- Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Müller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L.-E., Eils R. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38(8):970–979. doi: 10.1038/s41587-020-0602-4. - DOI - PubMed
-
- Lee H.K., Jung O., Hennighausen L. Activation of ACE2 and interferon-stimulated transcriptomes in human airway epithelium is curbed by Janus kinase inhibitors. bioRxiv. 2020 doi: 10.1101/2020.10.04.325415. 2020.10.04.325415. - DOI
-
- Sajuthi S.P., DeFord P., Li Y., Jackson N.D., Montgomery M.T., Everman J.L., Rios C.L., Pruesse E., Nolin J.D., Plender E.G., Wechsler M.E., Mak A.C.Y., Eng C., Salazar S., Medina V., Wohlford E.M., Huntsman S., Nickerson D.A., Germer S., Zody M.C., Abecasis G., Kang H.M., Rice K.M., Kumar R., Oh S., Rodriguez-Santana J., Burchard E.G., Seibold M.A. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020;11(1):5139. doi: 10.1038/s41467-020-18781-2. - DOI - PMC - PubMed
-
- Tembhre M.K., Parihar A.S., Sharma V.K., Imran S., Bhari N., Lakshmy R., Bhalla A. Enhanced expression of ACE2 in psoriatic skin and its upregulation in keratinocytes by interferon-gamma: implication of inflammatory milieu in skin tropism of SARS-CoV-2. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19670. - DOI - PubMed
-
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. - DOI - PMC - PubMed
-
- Fignani D., Licata G., Brusco N., Nigi L., Grieco G.E., Marselli L., Overbergh L., Gysemans C., Colli M.L., Marchetti P., Mathieu C., Eizirik D.L., Sebastiani G., Dotta F. SARS-CoV-2 receptor Angiotensin I-converting enzyme type 2 is expressed in human pancreatic islet β-cells and is upregulated by inflammatory stress. bioRxiv. 2020 doi: 10.1101/2020.07.23.208041. 2020.07.23.208041. - DOI - PMC - PubMed
-
- Verstockt B., Verstockt S., Abdu Rahiman S., Ke B.J., Arnauts K., Cleynen I., Sabino J., Ferrante M., Matteoli G., Vermeire S. Intestinal receptor of SARS-CoV-2 in inflamed IBD tissue seems downregulated by HNF4A in ileum and upregulated by interferon regulating factors in colon. J. Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa185. - DOI - PMC - PubMed
-
- Murphy R.C., Lai Y., Barrow K.A., Hamerman J.A., Lacy-Hulbert A., Piliponsky A.M., Ziegler S.F., Altemeier W.A., Debley J.S., Gharib S.A., Hallstrand T.S. Effects of asthma and human rhinovirus A16 on the expression of the SARS-CoV-2 entry factors in human airway epithelium. Am. J. Respir. Cell Mol. Biol. Sep. 2020;18 doi: 10.1165/rcmb.2020-0394LE. - DOI - PMC - PubMed
-
- Chen H., Liu W., Liu D., Zhao L., Yu J. SARS-CoV-2 activates lung epithelia cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients by single-cell sequencing. medRxiv. 2020 doi: 10.1101/2020.05.08.20096024. 2020.05.08.20096024. - DOI
-
- Turk C., Turk S., Temirci E.S., Malkan U.Y., Haznedaroglu İ C. In vitro analysis of the renin-angiotensin system and inflammatory gene transcripts in human bronchial epithelial cells after infection with severe acute respiratory syndrome coronavirus. J. Renin Angiotensin Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320928872. - DOI - PMC - PubMed
-
- Codo A.C., Davanzo G.G., Monteiro Ld.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo dos Santos K., Toledo-Teixeira D.A., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Vinolo M.A.R., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.07.007. - DOI - PMC - PubMed
-
- Onabajo O.O., Banday A.R., Yan W., Obajemu A., Stanifer M.L., Santer D.M., Florez-Vargas O., Piontkivska H., Vargas J., Kee C., Tyrrell D.L.J., Mendoza J.L., Boulant S., Prokunina-Olsson L. Interferons and viruses induce a novel primate-specific isoform dACE2 and not the SARS-CoV-2 receptor ACE2. bioRxiv. 2020 doi: 10.1101/2020.07.19.210955. 2020.07.19.210955. - DOI
-
- Blume C., Jackson C.L., Spalluto C.M., Legebeke J., Nazlamova L., Conforti F., Perotin-Collard J.-M., Frank M., Crispin M., Coles J., Thompson J., Ridley R.A., Dean L.S.N., Loxham M., Azim A., Tariq K., Johnston D., Skipp P.J., Djukanovic R., Baralle D., McCormick C., Davies D.E., Lucas J.S., Wheway G., Mennella V. A novel isoform of <em>ACE2</em> is expressed in human nasal and bronchial respiratory epithelia and is upregulated in response to RNA respiratory virus infection. bioRxiv. 2020 doi: 10.1101/2020.07.31.230870. 2020.07.31.230870. - DOI - PubMed
-
- Chen D.Y., Khan N., Close B.J., Goel R.K., Blum B., Tavares A.H., Kenney D., Conway H.L., Ewoldt J.K., Kapell S., Chitalia V.C., Crossland N.A., Chen C.S., Kotton D.N., Baker S.C., Connor J.H., Douam F., Emili A., Saeed M. SARS-CoV-2 desensitizes host cells to interferon through inhibition of the JAK-STAT pathway. bioRxiv. 2020 doi: 10.1101/2020.10.27.358259. - DOI
-
- Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.-P., Zhou J., Yuan S., Kok K.-H., To K.K.-W., Chan I.H.-Y., Zhang A.J., Sit K.-Y., Au W.-K., Yuen K.-Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020;71(6):1400–1409. doi: 10.1093/cid/ciaa410. - DOI - PMC - PubMed
-
- Gutiérrez-Chamorro L., Riveira-Muñoz E., Barrios C., Palau V., Massanella M., Garcia-Vidal E., Badia R., Pedreño S., Senserrich J., Rodríguez E., Clotet B., Cabrera C., Mitjà O., Crespo M., Pascual J., Riera M., Ballana E. SARS-CoV-2 infection suppresses ACE2 function and antiviral immune response in the upper respiratory tract of infected patients. bioRxiv. 2020 doi: 10.1101/2020.11.18.388850. 2020.11.18.388850. - DOI
-
- Pinto B.G.G., Oliveira A.E.R., Singh Y., Jimenez L., Goncalves A.N.A., Ogava R.L.T., Creighton R., Peron J.P.S., Nakaya H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa332. - DOI - PMC - PubMed
-
- Tseng C.T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M., Makino S., Packard M.M., Zaki S.R., Chan T.S., Peters C.J. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J. Virol. 2007;81(3):1162–1173. doi: 10.1128/jvi.01702-06. - DOI - PMC - PubMed
-
- Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., Liu S.S., Zhang N.N., Li X.F., Xiong R., Guo Y., Deng Y.Q., Huang W.J., Liu Q., Liu Q.M., Shen Y.L., Zhou Y., Yang X., Zhao T.Y., Fan C.F., Zhou Y.S., Qin C.F., Wang Y.C. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28(1):124–133. doi: 10.1016/j.chom.2020.05.020. e4. - DOI - PMC - PubMed
-
- Ahmetaj-Shala B., Vaja R., Atanur S.S., George P.M., Kirkby N.S., Mitchell J.A. Systemic analysis of putative SARS-CoV-2 entry and processing genes in cardiovascular tissues identifies a positive correlation of BSG with age in endothelial cells. bioRxiv. 2020 doi: 10.1101/2020.06.23.165324. 2020.06.23.165324. - DOI
-
- Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y., Shaw P.X., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191(5):755–760. doi: 10.1086/427811. - DOI - PMC - PubMed
-
- Ganier C., Du-Harpur X., Harun N., Wan B., Arthurs C., Luscombe N., Watt F., Lynch M. CD147 (<em>BSG</em>) but not <em>ACE2</em> expression is detectable in vascular endothelial cells within single cell RNA sequencing datasets derived from multiple tissues in healthy individuals. bioRxiv. 2020 doi: 10.1101/2020.05.29.123513. 2020.05.29.123513. - DOI
-
- Wang K., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., Wei D., Zhang Y., Sun X.-X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.-J., Chen R., Zhang H., Wang B., Zhu Y.-M., Nan G., Jiang J.-L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.-H., Zhang K., Miao J.-L., Cui H.-Y., Huang M., Zhang J., Fu L., Yang X.-M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.-F., Lu Y., Liu Y.-Y., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. - DOI - PMC - PubMed
-
- Amraie R., Napoleon M.A., Yin W., Berrigan J., Suder E., Zhao G., Olejnik J., Gummuluru S., Muhlberger E., Chitalia V., Rahimi N. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv. 2020 doi: 10.1101/2020.06.22.165803. 2020.06.22.165803. - DOI
-
- Gao C., Zeng J., Jia N., Stavenhagen K., Matsumoto Y., Zhang H., Li J., Hume A.J., Mühlberger E., van Die I., Kwan J., Tantisira K., Emili A., Cummings R.D. SARS-CoV-2 spike protein interacts with multiple innate immune receptors. bioRxiv. 2020 doi: 10.1101/2020.07.29.227462. 2020.07.29.227462. - DOI
-
- Gramberg T., Hofmann H., Möller P., Lalor P.F., Marzi A., Geier M., Krumbiegel M., Winkler T., Kirchhoff F., Adams D.H., Becker S., Münch J., Pöhlmann S. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340(2):224–236. doi: 10.1016/j.virol.2005.06.026. - DOI - PMC - PubMed
-
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U.S.A. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. - DOI - PMC - PubMed
-
- Marzi A., Gramberg T., Simmons G., Möller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pöhlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78(21):12090–12095. doi: 10.1128/jvi.78.21.12090-12095.2004. - DOI - PMC - PubMed
-
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78(11):5642–5650. doi: 10.1128/jvi.78.11.5642-5650.2004. - DOI - PMC - PubMed
-
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv. 2020 doi: 10.1101/2020.06.07.137802. 2020.06.07.137802. - DOI
-
- Daly J.L., Simonetti B., Antón-Plágaro C., Kavanagh Williamson M., Shoemark D.K., Simón-Gracia L., Klein K., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.06.05.134114. 2020.06.05.134114. - DOI - PubMed
-
- Liu L., Chopra P., Li X., Wolfert M.A., Tompkins S.M., Boons G.J. SARS-CoV-2 spike protein binds heparan sulfate in a length- and sequence-dependent manner. bioRxiv. 2020 doi: 10.1101/2020.05.10.087288. - DOI
-
- Ichimura T., Mori Y., Aschauer P., Padmanabha Das K.M., Padera R.F., Weins A., Nasr M.L., Bonventre J.V. KIM-1/TIM-1 is a receptor for SARS-CoV-2 in lung and kidney. medRxiv. 2020 doi: 10.1101/2020.09.16.20190694. - DOI
-
- Karmouty-Quintana H., Thandavarayan R.A., Keller S.P., Sahay S., Pandit L.M., Akkanti B. Emerging mechanisms of pulmonary vasoconstriction in SARS-CoV-2-Induced acute respiratory distress syndrome (ARDS) and potential therapeutic targets. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21218081. - DOI - PMC - PubMed
-
- Gu H., Xie Z., Li T., Zhang S., Lai C., Zhu P., Wang K., Han L., Duan Y., Zhao Z., Yang X., Xing L., Zhang P., Wang Z., Li R., Yu J.J., Wang X., Yang P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840. doi: 10.1038/srep19840. - DOI - PMC - PubMed
-
- Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F., Bai T., Han Z., Zhu J., Zhou H., Huang F., Li C., Lu H., Li N., Li D., Jin N., Penninger J.M., Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5(1):3594. doi: 10.1038/ncomms4594. - DOI - PMC - PubMed
-
- Treml B., Neu N., Kleinsasser A., Gritsch C., Finsterwalder T., Geiger R., Schuster M., Janzek E., Loibner H., Penninger J., Loeckinger A. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit. Care Med. 2010;38(2):596–601. doi: 10.1097/CCM.0b013e3181c03009. - DOI - PubMed
-
- Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. - DOI - PMC - PubMed
-
- Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P., Jin J., Bader M., Myohanen T., Garcia-Horsman J.A., Batlle D. Ang II (Angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting enzyme 2)-Independent. Hypertension. 2020;75(1):173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. - DOI - PMC - PubMed
-
- Huang F., Guo J., Zou Z., Liu J., Cao B., Zhang S., Li H., Wang W., Sheng M., Liu S., Pan J., Bao C., Zeng M., Xiao H., Qian G., Hu X., Chen Y., Chen Y., Zhao Y., Liu Q., Zhou H., Zhu J., Gao H., Yang S., Liu X., Zheng S., Yang J., Diao H., Cao H., Wu Y., Zhao M., Tan S., Guo D., Zhao X., Ye Y., Wu W., Xu Y., Penninger J.M., Li D., Gao G.F., Jiang C., Li L. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat. Commun. 2014;5:3595. doi: 10.1038/ncomms4595. - DOI - PMC - PubMed
-
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. - DOI - PMC - PubMed
-
- Liu N., Hong Y., Chen R.-G., Zhu H.-M. High rate of increased level of plasma Angiotensin II and its gender difference in COVID-19: an analysis of 55 hospitalized patients with COVID-19 in a single hospital, Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.04.27.20080432. 2020.04.27.20080432. - DOI
-
- Dudoignon E., Moreno N., Deniau B., Coutrot M., Longer R., Amiot Q., Mebazaa A., Pirracchio R., Depret F., Legrand M. Activation of the renin-angiotensin-aldosterone system is associated with Acute Kidney Injury in COVID-19. Anaesth. Crit. Care Pain Med. 2020;39(4):453–455. doi: 10.1016/j.accpm.2020.06.006. - DOI - PMC - PubMed
-
- Villard O., Morquin D., MOLINARI N., Raingeard I., Nagot N., Cristol J.-P., Jung B., Roubille C., Foulongne V., Fesler P., Lamure S., Taourel P., Konate A., Maria A.T.J., Makinson A., Bertchansky I., Larcher R., Klouche K., Le Moing V., Renard E., Guilpain P. The plasmatic aldosterone and C-reactive protein levels, and the severity of Covid-19: the Dyhor-19 study. J. Clin. Med. 2020;9(7):2315. doi: 10.3390/jcm9072315. - DOI - PMC - PubMed
-
- Rieder M., Wirth L., Pollmeier L., Jeserich M., Goller I., Baldus N., Schmid B., Busch H.J., Hofmann M., Kern W., Bode C., Duerschmied D., Lother A. Serum ACE-2, angiotensin II, and aldosterone levels are unchanged in patients with COVID-19. Am. J. Hypertens. 2020 doi: 10.1093/ajh/hpaa169. - DOI - PMC - PubMed
-
- Enes A., Pir P. Transcriptional response of signalling pathways to SARS-CoV-2 infection in normal human bronchial epithelial cells. bioRxiv. 2020 doi: 10.1101/2020.06.20.163006. 2020.06.20.163006. - DOI
-
- Kintscher U., Slagman A., Domenig O., Röhle R., Konietschke F., Poglitsch M., Möckel M. Plasma angiotensin peptide profiling and ACE2-Activity in COVID-19 patients treated with pharmacological blockers of the renin angiotensin system. Hypertension. 2020 doi: 10.1161/hypertensionaha.120.15841. - DOI - PMC - PubMed
-
- Schlicht K., Rohmann N., Geisler C., Hollstein T., Knappe C., Hartmann K., Schwarz J., Tran F., Schunk D., Junker R., Bahmer T., Rosenstiel P., Schulte D., Türk K., Franke A., Schreiber S., Laudes M. Circulating levels of soluble Dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infections. Int. J. Obes. 2020;44(11):2335–2338. doi: 10.1038/s41366-020-00689-y. - DOI - PMC - PubMed
-
- Goetz R., Joshi R., Chiles J., Scullin D., Wade R., Luckhardt T., Wells J. A remarkable response to angiotensin II therapy in severe SARS-COV-2 infection. Chest. 2020;158(4):A1012. doi: 10.1016/j.chest.2020.08.942. - DOI
-
- Leisman D.E., Mastroianni F., Fisler G., Shah S., Hasan Z., Narasimhan M., Taylor M.D., Deutschman C.S. Physiologic response to angiotensin II treatment for coronavirus disease 2019-Induced vasodilatory shock: a retrospective matched cohort study. Crit Care Explor. 2020;2(10):e0230. doi: 10.1097/cce.0000000000000230. - DOI - PMC - PubMed
-
- Grobe N., Weir N.M., Leiva O., Ong F.S., Bernstein K.E., Schmaier A.H., Morris M., Elased K.M. Identification of prolyl carboxypeptidase as an alternative enzyme for processing of renal angiotensin II using mass spectrometry. Am. J. Physiol., Cell Physiol. 2013;304(10):C945–953. doi: 10.1152/ajpcell.00346.2012. - DOI - PMC - PubMed
-
- Wysocki J., Ye M., Rodriguez E., González-Pacheco F.R., Barrios C., Evora K., Schuster M., Loibner H., Brosnihan K.B., Ferrario C.M., Penninger J.M., Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55(1):90–98. doi: 10.1161/hypertensionaha.109.138420. - DOI - PMC - PubMed
-
- Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M., Penninger J., Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52(9):783–792. doi: 10.1007/s40262-013-0072-7. - DOI - PubMed
-
- Hisatake S., Kiuchi S., Kabuki T., Oka T., Dobashi S., Ikeda T. Serum angiotensin-converting enzyme 2 concentration and angiotensin-(1-7) concentration in patients with acute heart failure patients requiring emergency hospitalization. Heart Vessels. 2017;32(3):303–308. doi: 10.1007/s00380-016-0877-z. - DOI - PubMed
-
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. - DOI - PMC - PubMed
-
- Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C., Neuhold S., Haider D., Stiasny K., Bergthaler A., Puchhammer-Stoeckl E., Mirazimi A., Montserrat N., Zhang H., Slutsky A.S., Penninger J.M. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8(11):1154–1158. doi: 10.1016/s2213-2600(20)30418-5. - DOI - PMC - PubMed
-
- Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., Liao S., Yang K., Li Q., Wan H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297(4):L631–640. doi: 10.1152/ajplung.90415.2008. - DOI - PubMed
-
- Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J., Humphries S.E., Hill M.R., Laurent G.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002;166(5):646–650. doi: 10.1164/rccm.2108086. - DOI - PubMed
-
- Gómez J., Albaiceta G.M., García-Clemente M., López-Larrea C., Amado-Rodríguez L., Lopez-Alonso I., Hermida T., Enriquez A.I., Herrero P., Melón S., Alvarez-Argüelles M.E., Boga J.A., Rojo-Alba S., Cuesta-Llavona E., Alvarez V., Lorca R., Coto E. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene. 2020;762 doi: 10.1016/j.gene.2020.145102. - DOI - PMC - PubMed
-
- Chiu R.W.K., Tang N.L.S., Hui D.S.C., Chung G.T.Y., Chim S.S.C., Chan K.C.A., Sung Y.-m., Chan L.Y.S., Tong Y.-k., Lee W.-s., Chan P.K.S., Lo Y.M.D. ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clin. Chem. 2004;50(9):1683–1686. doi: 10.1373/clinchem.2004.035436. - DOI - PMC - PubMed
-
- Itoyama S., Keicho N., Hijikata M., Quy T., Phi N.C., Long H.T., Ha L.D., Ban V.V., Matsushita I., Yanai H., Kirikae F., Kirikae T., Kuratsuji T., Sasazuki T. Identification of an alternative 5’-untranslated exon and new polymorphisms of angiotensin-converting enzyme 2 gene: lack of association with SARS in the Vietnamese population. Am. J. Med. Genet. A. 2005;136(1):52–57. doi: 10.1002/ajmg.a.30779. - DOI - PMC - PubMed
-
- Li W., Greenough T.C., Moore M.J., Vasilieva N., Somasundaran M., Sullivan J.L., Farzan M., Choe H. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 2004;78(20):11429–11433. doi: 10.1128/JVI.78.20.11429-11433.2004. - DOI - PMC - PubMed
-
- Golden J.W., Cline C.R., Zeng X., Garrison A.R., Carey B.D., Mucker E.M., White L.E., Shamblin J.D., Brocato R.L., Liu J., Babka A.M., Rauch H.B., Smith J.M., Hollidge B.S., Fitzpatrick C., Badger C.V., Hooper J.W. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. bioRxiv. 2020 doi: 10.1101/2020.07.09.195230. 2020.07.09.195230. - DOI - PMC - PubMed
-
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., Meyerholz D.K., Kirby P., Look D.C., Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. - DOI - PMC - PubMed
-
- Oladunni F.S., Park J.-G., Tamayo P.P., Gonzalez O., Akhter A., Allué-Guardia A., Olmo-Fontánez A., Gautam S., Garcia-Vilanova A., Ye C., Chiem K., Headley C., Dwivedi V., Parodi L.M., Alfson K.J., Staples H.M., Schami A., Garcia J.I., Whigham A., Platt R.N., Gazi M., Martinez J., Chuba C., Earley S., Rodriguez O.H., Mdaki S.D., Kavelish K.N., Escalona R., Hallam C.R.A., Christie C., Patterson J.L., Anderson T.J.C., Carrion R., Dick E.J., Hall-Ursone S., Schlesinger L.S., Kaushal D., Giavedoni L.D., Alvarez X., Turner J., Martinez-Sobrido L., Torrelles J.B. Lethality of SARS-CoV-2 infection in K18 human angiotensin converting enzyme 2 transgenic mice. bioRxiv. 2020 doi: 10.1101/2020.07.18.210179. 2020.07.18.210179. - DOI - PMC - PubMed
-
- Huang L., Sexton D.J., Skogerson K., Devlin M., Smith R., Sanyal I., Parry T., Kent R., Enright J., Wu Q.L., Conley G., DeOliveira D., Morganelli L., Ducar M., Wescott C.R., Ladner R.C. Novel peptide inhibitors of angiotensin-converting enzyme 2. J. Biol. Chem. 2003;278(18):15532–15540. doi: 10.1074/jbc.M212934200. - DOI - PubMed
-
- Dales N.A., Gould A.E., Brown J.A., Calderwood E.F., Guan B., Minor C.A., Gavin J.M., Hales P., Kaushik V.K., Stewart M., Tummino P.J., Vickers C.S., Ocain T.D., Patane M.A. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J. Am. Chem. Soc. 2002;124(40):11852–11853. doi: 10.1021/ja0277226. - DOI - PubMed
-
- Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., Patane M.A., Pantoliano M.W. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. - DOI - PMC - PubMed
-
- Pedersen K.B., Sriramula S., Chhabra K.H., Xia H., Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(5):R1293–1299. doi: 10.1152/ajpregu.00339.2011. - DOI - PMC - PubMed
-
- Ye M., Wysocki J., Gonzalez-Pacheco F.R., Salem M., Evora K., Garcia-Halpin L., Poglitsch M., Schuster M., Batlle D. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60(3):730–740. doi: 10.1161/HYPERTENSIONAHA.112.198622. - DOI - PMC - PubMed
-
- Hernández Prada J.A., Ferreira A.J., Katovich M.J., Shenoy V., Qi Y., Santos R.A., Castellano R.K., Lampkins A.J., Gubala V., Ostrov D.A., Raizada M.K. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51(5):1312–1317. doi: 10.1161/hypertensionaha.107.108944. - DOI - PubMed
-
- Haber P.K., Ye M., Wysocki J., Maier C., Haque S.K., Batlle D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension. 2014;63(4):774–782. doi: 10.1161/hypertensionaha.113.02856. - DOI - PMC - PubMed
-
- Qi Y., Zhang J., Cole-Jeffrey C.T., Shenoy V., Espejo A., Hanna M., Song C., Pepine C.J., Katovich M.J., Raizada M.K. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62(4):746–752. doi: 10.1161/hypertensionaha.113.01337. - DOI - PMC - PubMed
-
- Qaradakhi T., Gadanec L.K., McSweeney K.R., Tacey A., Apostolopoulos V., Levinger I., Rimarova K., Egom E.E., Rodrigo L., Kruzliak P., Kubatka P., Zulli A. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin. Exp. Pharmacol. Physiol. 2020;47(5):751–758. doi: 10.1111/1440-1681.13251. - DOI - PubMed
-
- Senthil Kumar K.J., Gokila Vani M., Wang C.S., Chen C.C., Chen Y.C., Lu L.P., Huang C.H., Lai C.S., Wang S.Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants (Basel) 2020;9(6) doi: 10.3390/plants9060770. - DOI - PMC - PubMed
-
- Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Thi Phuong Loan H., Triet N.T., Anh T.T.V., Quy P.T., Tat P.V., Hue N.V., Quang D.T., Trung N.T., Tung V.T., Huynh L.K., Nhung N.T.A. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5(14):8312–8320. doi: 10.1021/acsomega.0c00772. - DOI - PMC - PubMed
-
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. e7. - DOI - PMC - PubMed
-
- Iwanaga N., Cooper L., Rong L., Beddingfield B., Crabtree J., Tripp R.A., Kolls J.K. Novel ACE2-IgG1 fusions with improved activity against SARS-CoV2. bioRxiv. 2020 doi: 10.1101/2020.06.15.152157. - DOI
-
- Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.-M., Zeng Q., Tahan S., Droit L., Ilagan M.X.G., Tartell M.A., Amarasinghe G., Henderson J.P., Miersch S., Ustav M., Sidhu S., Virgin H.W., Wang D., Ding S., Corti D., Theel E.S., Fremont D.H., Diamond M.S., Whelan S.P.J. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020;28(3):475–485. doi: 10.1016/j.chom.2020.06.021. e5. - DOI - PMC - PubMed
-
- Monteil V., Dyczynski M., Lauschke V.M., Kwon H., Wirnsberger G., Youhanna S., Zhang H., A S.S., Hurtado Del Pozo C., Horn M., Montserrat N., Penninger J.M., Mirazimi A. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2020:e13426. doi: 10.15252/emmm.202013426. - DOI - PMC - PubMed
-
- Karoyan P., Vieillard V., Odile E., Denis A., Gómez-Morales L., Grondin P., Lequin O. An hACE2 peptide mimic blocks SARS-CoV-2 pulmonary cell infection. bioRxiv. 2020 doi: 10.1101/2020.08.24.264077. 2020.08.24.264077. - DOI
-
- Guo L., Bi W., Wang X., Xu W., Yan R., Zhang Y., Zhao K., Li Y., Zhang M., Cai X., Jiang S., Xie Y., Zhou Q., Lu L., Dang B. Engineered trimeric ACE2 binds viral spike protein and locks it in “Three-up” conformation to potently inhibit SARS-CoV-2 infection. Cell Res. 2020 doi: 10.1038/s41422-020-00438-w. - DOI - PMC - PubMed
-
- Linsky T.W., Vergara R., Codina N., Nelson J.W., Walker M.J., Su W., Barnes C.O., Hsiang T.-Y., Esser-Nobis K., Yu K., Reneer Z.B., Hou Y.J., Priya T., Mitsumoto M., Pong A., Lau U.Y., Mason M.L., Chen J., Chen A., Berrocal T., Peng H., Clairmont N.S., Castellanos J., Lin Y.-R., Josephson-Day A., Baric R.S., Fuller D.H., Walkey C.D., Ross T.M., Swanson R., Bjorkman P.J., Gale M., Blancas-Mejia L.M., Yen H.-L., Silva D.-A. De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2. Science. 2020:eabe0075. doi: 10.1126/science.abe0075. - DOI - PMC - PubMed
-
- Glasgow A., Glasgow J., Limonta D., Solomon P., Lui I., Zhang Y., Nix M.A., Rettko N.J., Lim S.A., Zha S., Yamin R., Kao K., Rosenberg O.S., Ravetch J.V., Wiita A.P., Leung K.K., Zhou X.X., Hobman T.C., Kortemme T., Wells J.A. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.07.31.231746. - DOI - PMC - PubMed
-
- Li Y., Wang H., Tang X., Fang S., Ma D., Du C., Wang Y., Pan H., Yao W., Zhang R., Zou X., Zheng J., Xu L., Farzan M., Zhong G. SARS-CoV-2 and three related coronaviruses utilize multiple ACE2 orthologs and are potently blocked by an improved ACE2-Ig. J. Virol. 2020 doi: 10.1128/jvi.01283-20. - DOI - PMC - PubMed
-
- Higuchi Y., Suzuki T., Arimori T., Ikemura N., Kirita Y., Ohgitani E., Mazda O., Motooka D., Nakamura S., Matsuura Y., Matoba S., Okamoto T., Takagi J., Hoshino A. High affinity modified ACE2 receptors prevent SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.09.16.299891. 2020.09.16.299891. - DOI
-
- Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79(10):5900–5906. doi: 10.1128/jvi.79.10.5900-5906.2005. - DOI - PMC - PubMed
-
- Chen X., Li R., Pan Z., Qian C., Yang Y., You R., Zhao J., Liu P., Gao L., Li Z., Huang Q., Xu L., Tang J., Tian Q., Yao W., Hu L., Yan X., Zhou X., Wu Y., Deng K., Zhang Z., Qian Z., Chen Y., Ye L. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell. Mol. Immunol. 2020;17(6):647–649. doi: 10.1038/s41423-020-0426-7. - DOI - PMC - PubMed
-
- Ejemel M., Li Q., Hou S., Schiller Z.A., Tree J.A., Wallace A., Amcheslavsky A., Kurt Yilmaz N., Buttigieg K.R., Elmore M.J., Godwin K., Coombes N., Toomey J.R., Schneider R., Ramchetty A.S., Close B.J., Chen D.Y., Conway H.L., Saeed M., Ganesa C., Carroll M.W., Cavacini L.A., Klempner M.S., Schiffer C.A., Wang Y. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020;11(1):4198. doi: 10.1038/s41467-020-18058-8. - DOI - PMC - PubMed
-
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., Gong Y., Xiao H., Fan Z., Tan S., Wu G., Tan W., Lu X., Fan C., Wang Q., Liu Y., Zhang C., Qi J., Gao G.F., Gao F., Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274–1278. doi: 10.1126/science.abc2241. - DOI - PMC - PubMed
-
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Martinez D.R., Williamson L.E., Chen E.C., Jones T., Day S., Myers L., Hassan A.O., Kafai N.M., Winkler E.S., Fox J.M., Shrihari S., Mueller B.K., Meiler J., Chandrashekar A., Mercado N.B., Steinhardt J.J., Ren K., Loo Y.M., Kallewaard N.L., McCune B.T., Keeler S.P., Holtzman M.J., Barouch D.H., Gralinski L.E., Baric R.S., Thackray L.B., Diamond M.S., Carnahan R.H., Crowe J.E., Jr. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449. doi: 10.1038/s41586-020-2548-6. - DOI - PMC - PubMed
-
- Byrnes J.R., Zhou X.X., Lui I., Elledge S.K., Glasgow J.E., Lim S.A., Loudermilk R.P., Chiu C.Y., Wang T.T., Wilson M.R., Leung K.K., Wells J.A. Competitive SARS-CoV-2 serology reveals most antibodies targeting the spike receptor-binding domain compete for ACE2 binding. mSphere. 2020;5(5) doi: 10.1128/mSphere.00802-20. - DOI - PMC - PubMed
-
- Wang N., Han S., Liu R., Meng L., He H., Zhang Y., Wang C., Lv Y., Wang J., Li X., Ding Y., Fu J., Hou Y., Lu W., Ma W., Zhan Y., Dai B., Zhang J., Pan X., Hu S., Gao J., Jia Q., Zhang L., Ge S., Wang S., Liang P., Hu T., Lu J., Wang X., Zhou H., Ta W., Wang Y., Lu S., He L. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. bioRxiv. 2020 doi: 10.1101/2020.06.22.164665. 2020.06.22.164665. - DOI - PMC - PubMed
-
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. - DOI - PMC - PubMed
-
- Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85(3):551–555. doi: 10.1016/j.antiviral.2009.12.001. - DOI - PMC - PubMed
![Fig. 1 The renin angiotensin system (RAS). The classical RAS consists of the breakdown of Angiotensin I (Ang-I) into Ang-II via ACE, which can bind either to the AT1 (angiotensin type 1) or the AT2 (angiotensin type 2) receptor. Ang-II has a higher affinity for the AT1 receptor. The non-classical RAS consists of conversion of Ang-I into Ang-(1-9) and Ang-II into Ang-(1-7) by ACE2. Ang-(1-7) stimulates the Mas receptor. Bradykinin and [des-Arg9]-bradykinin are degraded by ACE and ACE2, respectively, into pharmacologically inactive peptides.](/Images/Figures/17_4045.jpeg)