The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out?
Affiliations
Affiliations
- Department of Physiology, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait.
Abstract
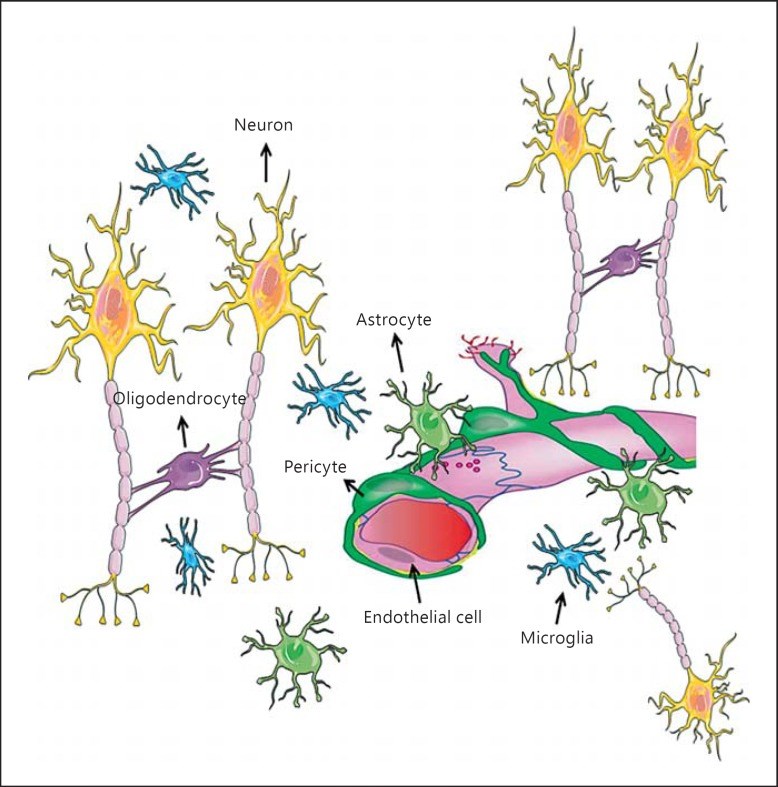
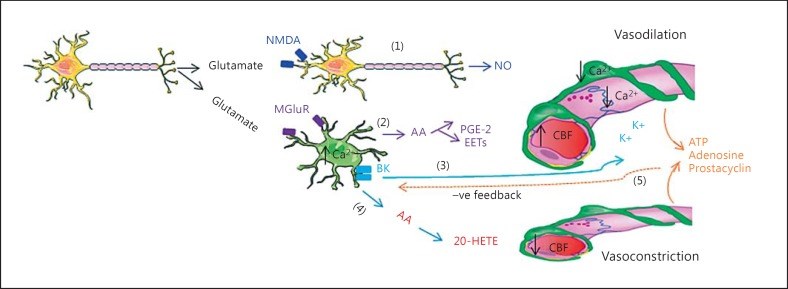
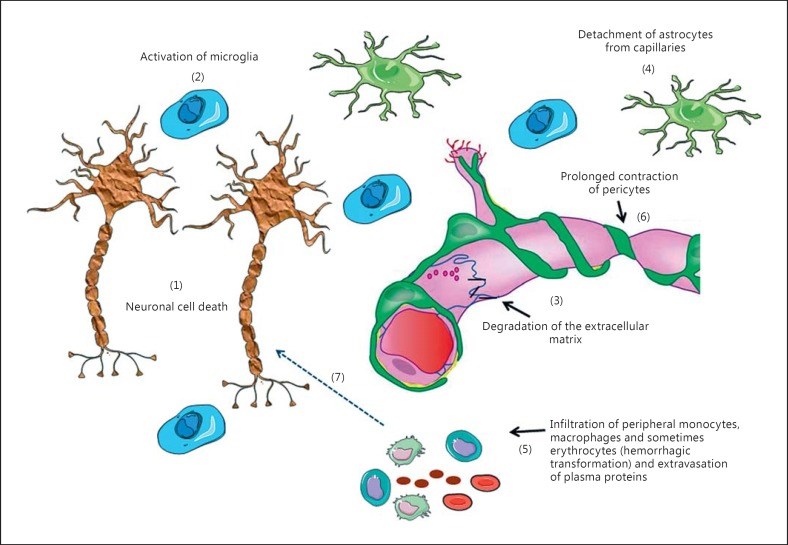
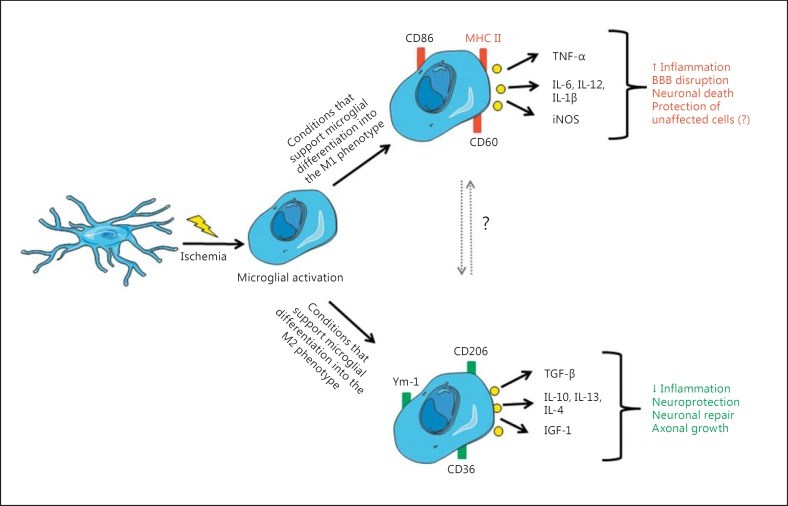
Paracrine signaling in the neurovascular unit (NVU) is aimed to adjust the supply of oxygen and nutrients to metabolic demands of the brain in a feed-forward manner. Cerebral ischemia (CI) severely disrupts this homeostatic mechanism and also causes activation of microglia and resident macrophages in the brain. Contradictory data exist on the time pattern of microglial activation and polarization during CI, on molecular mechanisms that trigger them and on effects of microglia-derived cytokines on brain cells. It appears that conditions that occur during transient ischemia or in the penumbra of focal ischemia in vivo or equivalent conditions in vitro trigger polarization of resting microglia/macrophages into the M2 phenotype, which mainly exerts anti-inflammatory and protective effects in the brain, while prolonged ischemia with abundant necrosis promotes microglial polarization into the M1 phenotype. During the later stages of recovery, microglia that polarized initially into the M2 phenotype can shift into the M1 phenotype. Thus, it appears that cells with both phenotypes are present in the affected area, but their relative amount changes in time and probably depends on the proximity to the ischemic core. It was assumed that cells with the M1 phenotype exert detrimental effects on neurons and contribute to the blood-brain barrier opening. Several M1 phenotype-specific cytokines exert protective effects on astrocytes, which could be important for reactive gliosis occurring after ischemia. Thus, whether or not suppression of microglial activity after CI is beneficial for neurological outcome still remains unclear and current evidence suggests that no simple answer could be given to this question.
Figures
Similar articles
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J.Stroke. 2012 Nov;43(11):3063-70. doi: 10.1161/STROKEAHA.112.659656. Epub 2012 Aug 28.PMID: 22933588
Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL.J Neurochem. 2016 Oct;139(1):96-105. doi: 10.1111/jnc.13751. Epub 2016 Sep 9.PMID: 27470181 Free PMC article.
Barakat R, Redzic Z.Fluids Barriers CNS. 2015 Feb 28;12:6. doi: 10.1186/s12987-015-0002-1. eCollection 2015.PMID: 25866619 Free PMC article.
Regulation of microglial activation in stroke.
Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F.Acta Pharmacol Sin. 2017 Apr;38(4):445-458. doi: 10.1038/aps.2016.162. Epub 2017 Mar 6.PMID: 28260801 Free PMC article. Review.
Diversity and plasticity of microglial cells in psychiatric and neurological disorders.
Nakagawa Y, Chiba K.Pharmacol Ther. 2015 Oct;154:21-35. doi: 10.1016/j.pharmthera.2015.06.010. Epub 2015 Jun 27.PMID: 26129625 Review.
Cited by
Common Signaling Pathways Involved in Alzheimer's Disease and Stroke: Two Faces of the Same Coin.
Das TK, Ganesh BP, Fatima-Shad K.J Alzheimers Dis Rep. 2023 May 12;7(1):381-398. doi: 10.3233/ADR-220108. eCollection 2023.PMID: 37220617 Free PMC article. Review.
Post-traumatic Stress Disorder: Focus on Neuroinflammation.
Li J, Tong L, Schock BC, Ji LL.Mol Neurobiol. 2023 Jul;60(7):3963-3978. doi: 10.1007/s12035-023-03320-z. Epub 2023 Apr 1.PMID: 37004607 Review.
New insights in ferroptosis: Potential therapeutic targets for the treatment of ischemic stroke.
Wei Z, Xie Y, Wei M, Zhao H, Ren K, Feng Q, Xu Y.Front Pharmacol. 2022 Nov 8;13:1020918. doi: 10.3389/fphar.2022.1020918. eCollection 2022.PMID: 36425577 Free PMC article. Review.
Yakupova EI, Maleev GV, Krivtsov AV, Plotnikov EY.Exp Biol Med (Maywood). 2022 Jun;247(11):958-971. doi: 10.1177/15353702221080130. Epub 2022 Feb 26.PMID: 35220781 Free PMC article.
Yang L, Yu X, Zhang Y, Liu N, Xue X, Fu J.Pediatr Res. 2022 Dec;92(6):1543-1554. doi: 10.1038/s41390-021-01924-6. Epub 2022 Feb 26.PMID: 35220399 Free PMC article.
KMEL References
References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Bertini G, Bramanti P, Constantin G, et al. New players in the neurovascular unit: insights from experimental and clinical epilepsy. Neurochem Int. 2013;63:652–659. - PubMed
-
- Lok J, Gupta P, Guo S, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. - PubMed
-
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. - PubMed
-
- Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. - PubMed
-
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. - PubMed
-
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:73–81. - PubMed
-
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. - PubMed
-
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Sakimoto S, Kidoya H, Naito H, et al. A role for endothelial cells in promoting the maturation of astrocytes through the apelin/APJ system in mice. Development. 2012;139:1327–1335. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Lee HS, Han J, Bai HJ, et al. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009;276:4622–4635. - PubMed
-
- Durafourt BA, Moore CS, Zammit DA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. - PubMed
-
- Smith JA, Das A, Ray SK, et al. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. - PubMed
-
- Becher B, Prat A, Antel JP. Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. - PubMed
-
- Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84:49–59. - PubMed
-
- Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev. 2011;91:461–553. - PubMed
-
- Smith JA, Das A, Ray SK, et al. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. - PubMed
-
- Hung J, Chansard M, Ousman SS, et al. Activation of microglia by neuronal activity: results from a new in vitro paradigm based on neuronal-silicon interfacing technology. Brain Behav Immun. 2010;24:31–40. - PubMed
-
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. - PubMed
-
- Nakka VP, Gusain A, Mehta SL, et al. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. - PubMed
-
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. - PubMed
-
- Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol (Oxf) 2013;208:148–165. - PubMed
-
- Del Zoppo GJ. Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke. 2013;44:263–269. - PubMed
-
- Del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. - PubMed
-
- Del Zoppo GJ, Milner R, Mabuchi T, et al. Vascular matrix adhesion and the blood-brain barrier. Biochem Soc Trans. 2006;34:1261–1266. - PubMed
-
- Del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. - PubMed
-
- Boscia F, D'Avanzo C, Pannaccione A, et al. New roles of NCX in glial cells: activation of microglia in ischemia and differentiation of oligodendrocytes. Adv Exp Med Biol. 2013;961:307–316. - PubMed
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
-
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. - PubMed
-
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
-
- Kofler J, Wiley CA. Microglia: key innate immune cells of the brain. Toxicol Pathol. 2011;39:103–114. - PubMed
-
- Michelucci A, Heurtaux T, Grandbarbe L, et al. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. - PubMed
-
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. - PubMed
-
- Olah M, Biber K, Vinet J, et al. Microglia phenotype diversity. CNS Neurol Disord Drug Targets. 2011;10:108–118. - PubMed
-
- Corti R, Hutter R, Badimon JJ, et al. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J Thromb Thrombolysis. 2004;17:35–44. - PubMed
-
- Zameer A, Hoffman SA. Increased ICAM-1 and VCAM-1 expression in the brains of autoimmune mice. J Neuroimmunol. 2003;142:67–74. - PubMed
-
- Spitzbarth I, Baumgartner W, Beineke A. The role of pro- and anti-inflammatory cytokines in the pathogenesis of spontaneous canine CNS diseases. Vet Immunol Immunopathol. 2012;15(147):6–24. - PubMed
-
- Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3770. - PubMed
-
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. - PubMed
-
- Lehrmann E, Kiefer R, Finsen B, et al. Cytokines in cerebral ischemia: expression of transforming growth factor beta-1 (TGF-beta 1) mRNA in the postischemic adult rat hippocampus. Exp Neurol. 1995;131:114–123. - PubMed
-
- Zhu Y, Roth-Eichhorn S, Braun N, et al. The expression of transforming growth factor-beta1 (TGF-beta1) in hippocampal neurons: a temporary upregulated protein level after transient forebrain ischemia in the rat. Brain Res. 2000;866:286–298. - PubMed
-
- Imai F, Suzuki H, Oda J, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab. 2007;27:488–500. - PubMed
-
- Lee GA, Lin CH, Jiang HH, et al. Microglia-derived glial cell line-derived neurotrophic factor could protect Sprague-Dawley rat astrocyte from in vitro ischemia-induced damage. Neurosci Lett. 2004;356:111–114. - PubMed
-
- Chu LF, Wang WT, Ghanta VK, et al. Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-κB signaling pathway. Brain Res. 2008;1239:24–35. - PubMed
-
- Lu YZ, Lin CH, Cheng FC, et al. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373:159–164. - PubMed
-
- Redzic ZB, Rabie T, Sutherland BA, et al. Differential effects of paracrine factors on the survival of cells of the neurovascular unit during oxygen glucose deprivation. Int J Stroke. 2015;10:407–414. - PubMed
-
- Goss CE, Bednar MM, Howard DB, et al. Transforming growth factor-beta 1 reduces infarct size after experimental cerebral ischemia in a rabbit model. Stroke. 1993;24:558–562. - PubMed
-
- Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. J Intensive Care Med. 2008;23:236–249. - PubMed
-
- Sandhu JK, Gardaneh M, Iwasiow R, et al. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis. 2009;33:405–414. - PubMed
-
- Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. - PubMed
-
- Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. - PubMed
-
- Tilg H, Trehu E, Atkins MB, et al. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. - PubMed
-
- Ali C, Nicole O, Docagne F, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. - PubMed
-
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. - PubMed
-
- Yamashita T, Sawamoto K, Suzuki S, et al. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. - PubMed