Effect of various blood glucose levels on regional FDG uptake in the brain
Affiliations
Affiliations
- Department of Nuclear Medicine, Faculty of Medicine, Mubarak Al-Kabeer Hospital, Kuwait University, Kuwait.
- Department of Community Medicine and Behavioral Sciences, Faculty of Medicine, Kuwait University, Kuwait.
- Department of Nuclear Medicine, Faculty of Medicine, Trakya University, Edirne, Turkey.
Abstract
Objectives: Studies have mainly assessed the effect of hyperglycemia on 18F-fluorodeoxyglucose (FDG) uptake in the brain. In this study, we assessed the FDG uptake of the brain not only in normo- and hyperglycemia but also in hypoglycemia to compare the effect of various blood glucose levels on regional FDG uptake in the brain.
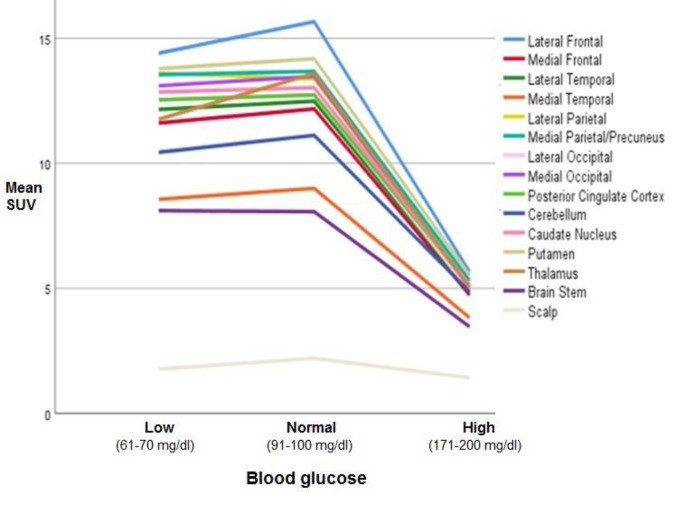
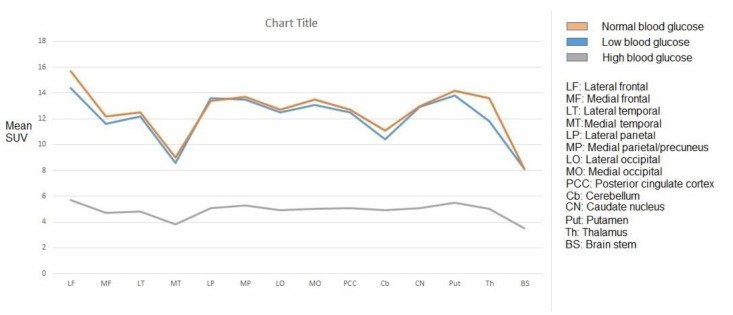
Methods: This retrospective study was conducted on whole-body FDG positron emission tomography/computed tomography (PET/CT) images including the brain. The inclusion criteria included adult patients with no known history of diseases or symptoms affecting the brain, lack of abnormal brain findings on both PET and CT images, no image artifacts, and lack of any factors affecting brain FDG uptake. Maximum standardized uptake values (SUVmax) were measured in the lateral and medial frontal, temporal, parietal, and occipital cortices, lateral cerebellar cortex, posterior cingulate cortex, caudate nucleus, putamen, thalamus, brain stem (BS), and scalp in patients with normal (91-100 mg/dl), low (61-70 mg/dl), and high (171-200 mg/dl) blood glucose (BG) levels. Mean SUVmax of the brain regions for each BG range was calculated and statistically analyzed.
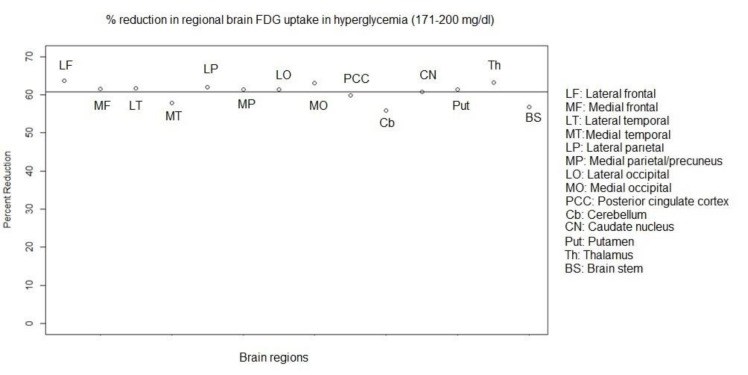
Results: In all BG levels, FDG uptake was at the highest level in the lateral frontal cortex and lowest level in the medial temporal cortex (MTC) and BS. The SUVmax in all assessed brain regions was significantly lower in hyperglycemia (P<0.001). However, this value was not significantly different in hypoglycemia (P>0.05) as compared to that in normoglycemia. At the BG range of 171-200 mg/dl, hyperglycemia-induced reduction in regional SUVmax had a range of 55.9-63.7% (60%±2.4%). This reduction was below 60% in the MTC, cerebellum, and BS and above 60% in other regions. Scalp activity was lower in hyperglycemia (P<0.001) and not different in hypoglycemia (P>0.05) as compared to normoglycemia.
Conclusion: The FDG uptake appears to be at the highest level in the lateral frontal cortex and the lowest level in the MTC and BS in normo-, hypo-, and hyperglycemia. Hyperglycemia-induced reduction in FDG uptake was approximately the same as that in various regions of the brain. However, the MTC, cerebellum, and BS may be slightly less affected than the other regions. Hypoglycemia does not seem to have a significant effect on FDG uptake in the brain.
Keywords: Blood glucose; Brain; FDG; PET; SUV.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
Sarikaya I, Sarikaya A, Sharma P.J Nucl Med Technol. 2019 Dec;47(4):313-318. doi: 10.2967/jnmt.119.226969. Epub 2019 Jun 10.PMID: 31182660
Pianou NK, Stavrou PZ, Vlontzou E, Rondogianni P, Exarhos DN, Datseris IE.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7.PMID: 30843003
Viglianti BL, Wong KK, Wimer SM, Parameswaran A, Nan B, Ky C, Townsend DM, Rubello D, Frey KA, Gross MD.Biomed Pharmacother. 2017 Apr;88:1038-1045. doi: 10.1016/j.biopha.2017.01.166. Epub 2017 Feb 7.PMID: 28192877 Free PMC article.
Kawasaki K, Ishii K, Saito Y, Oda K, Kimura Y, Ishiwata K.Ann Nucl Med. 2008 Apr;22(3):191-200. doi: 10.1007/s12149-007-0099-7. Epub 2008 May 23.PMID: 18498034
Guan ZW, Xu BX, Wang RM, Sun L, Tian JH.Hell J Nucl Med. 2013 May-Aug;16(2):97-102. doi: 10.1967/s002449910084. Epub 2013 May 20.PMID: 23687644 Review.
Cited by
Weise CM, Chen K, Chen Y, Devadas V, Su Y, Reiman EM.Front Aging Neurosci. 2022 Nov 22;14:1031189. doi: 10.3389/fnagi.2022.1031189. eCollection 2022.PMID: 36570534 Free PMC article.
Lei H, Hu R, Luo G, Yang T, Shen H, Deng H, Chen C, Zhao H, Liu J.Front Hum Neurosci. 2022 Jan 6;15:755017. doi: 10.3389/fnhum.2021.755017. eCollection 2021.PMID: 35069149 Free PMC article. Review.
Multimodality In Vivo Imaging of Perfusion and Glycolysis in a Rat Model of C6 Glioma.
Qi Q, Fox MS, Lim H, Bartha R, Scholl TJ, Hoffman L, Lee TY, Thiessen JD.Mol Imaging Biol. 2021 Aug;23(4):516-526. doi: 10.1007/s11307-021-01585-1. Epub 2021 Feb 3.PMID: 33534038
KMEL References
References
-
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5. - PubMed
-
- Büsing KA, Schönberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013;40:206–13. - PubMed
-
- Keramida G, Dizdarevic S, Bush J, Peters AM. Quantification of tumor (18) F-FDG uptake: Normalise to blood glucose or scale to liver uptake? Eur Radiol. 2015:252701–8. - PubMed
-
- Claeys J, Mertens K, Asseler YD. Normoglycemic plasma glucose levels affect F-18FDG uptake in the brain. Ann Nucl Med. 2010;24:501–505. - PubMed
-
- Sarikaya I, Sarikaya A, Sharma P. Assessing effect of various blood glucose levels on 18F-FDG activity in the brain, liver and blood pool. J Nucl Med Technol. 2019 Accepted for publication. - PubMed
-
- Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. European Association of Nuclear Medicine Neuroimaging Committee EANM procedure guidelines for PET brain imaging using [18F] FDG version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10. - PubMed
-
- Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging Version 10. approved February 8, 2009.
-
- Ivançević V, Alavi A, Souder E, Mozley PD, Gur RE, Bénard F, et al. Regional cerebral glucose metabolism in healthy volunteers determined by fluordeoxyglucose positron emission tomography: appearance and variance in the transaxial, coronal, and sagittal planes. Clin Nucl Med. 2000;25:596–602. - PubMed
-
- Pourhassan Shamchi S, Khosravi M, Taghvaei R, Zirakchian Zadeh M, Paydary K, Emamzadehfard S, et al. Normal patterns of regional brain (18) F-FDG uptake in normal aging. Hell J Nucl Med. 2018;21:175–180. - PubMed
-
- Kim IJ, Kim SJ, Kim YK. Age- and sex-associated changes in cerebral glucose metabolism in normal healthy subjects: statistical parametric mapping analysis of F- 18fluorodeoxyglucose brain positron emission tomography. Acta Radiol. 2009;50:1169–74. - PubMed
-
- Kawasaki K, Ishii K, Saito Y, Oda K, Kimura Y, Ishiwata K. Influence of mild hyperglycemia on cerebral FDG distribution patterns calculated by statistical mapping. Ann Nucl Med. 2008;22:191–200. - PubMed
-
- Ishibashi K, Onishi A, Fujiwara Y, Ishiwata K, Ishii K. Plasma Glucose Levels Affect cerebral 18F-FDG distribution in cognitively normal subjects with diabetes. Clin Nucl Med. 2016;41:e274–80. - PubMed
-
- Hou WK, Xian YX, Zhang L, Lai H, Hou XG, Xu YX, et al. Influence of blood glucose on the expression of glucose trans-porter proteins 1 and 3 in the brain of diabetic rats. Chin Med J (Engl). 2007;20:1704–9. - PubMed
-
- Márián T, Balkay L, Fekete I, Lengyel Z, Veress G, Esik O, et al. Hypoglycemia activates compensatory mechanism of glucose metabolism of brain. Acta Biol Hung. 2001;52:35–45. - PubMed
-
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–63. - PubMed
-
- Sarikaya I, Sarikaya A, Elgazzar AH. Current Status of (18) F-FDG PET Brain Imaging in Patients with Dementia. J Nucl Med Technol. 2018;46:362–367. - PubMed
-
- Sorokin J, Saboury B, Ahn JA, Moghbel M, Basu S, Alavi A. Adverse functional effects of chemotherapy on whole-brain metabolism: a PET/CT quantitative analysis of FDG metabolic pattern of the "chemo-brain". Clin Nucl Med. 2014;39:35–9. - PubMed
-
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–11. - PubMed