Effect of Helmet Noninvasive Ventilation vs Usual Respiratory Support on Mortality Among Patients With Acute Hypoxemic Respiratory Failure Due to COVID-19: The HELMET-COVID Randomized Clinical Trial
Yaseen M Arabi 1 2 3, Sara Aldekhyl 1 2 3, Saad Al Qahtani 1 2 3, Hasan M Al-Dorzi 1 2 3, Sheryl Ann Abdukahil 1 2 3, Mohammed Khulaif Al Harbi 2 3 4, Eman Al Qasim 1 2 3, Ayman Kharaba 5, Talal Albrahim 6, Mohammed S Alshahrani 7, Abdulrahman A Al-Fares 8, Ali Al Bshabshe 9, Ahmed Mady 10 11, Zainab Al Duhailib 12, Haifa Algethamy 13, Jesna Jose 1 3 14, Mohammed Al Mutairi 2 3 15, Omar Al Zumai 2 3 15, Hussain Al Haji 2 3 15, Ahmed Alaqeily 2 3 15, Zohair Al Aseri 16, Awad Al-Omari 17, Abdulaziz Al-Dawood 1 2 3, Haytham Tlayjeh 1 2 3; Saudi Critical Care Trials Group
Affiliations
Affiliations
- 1Intensive Care Department, Ministry of National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia.
- 2King Abdullah International Medical Research Center, Riyadh, Kingdom of Saudi Arabia.
- 3King Saud bin Abdulaziz University for Health Sciences, Riyadh, Kingdom of Saudi Arabia.
- 4Department of Anesthesia, Ministry of National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia.
- 5Pulmonary and Critical Care Departments, King Fahad Hospital, Madinah, Kingdom of Saudi Arabia.
- 6Department of Critical Care, King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Al Khobar, Kingdom of Saudi Arabia.
- 7Department of Emergency and Critical Care, King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Al Khobar, Kingdom of Saudi Arabia.
- 8Department of Anesthesia, Critical Care Medicine and Pain Medicine, Al-Amiri Hospital, Ministry of Health, Kuwait, Kuwait.
- 9Department of Critical Care Medicine, King Khalid University, Aseer Central Hospital, Abha, Kingdom of Saudi Arabia.
- 10Intensive Care Department, King Saud Medical City, Riyadh, Kingdom of Saudi Arabia.
- 11College of Medicine, Tanta University, Tanta, Egypt.
- 12Adult Critical Care Medicine Department, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia.
- 13Department of Anesthesia and Critical Care, King Abdulaziz University, King Abdulaziz University Hospital, Jeddah, Kingdom of Saudi Arabia.
- 14Department of Bioinformatics and Biostatistics, King Abdullah International Medical Research Center, Riyadh, Kingdom of Saudi Arabia.
- 15Respiratory Services Department, Ministry of National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia.
- 16Emergency and Intensive Care Departments, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia.
- 17Alfaisal University, Critical Care and Infectious Disease and Infection Control Departments, Dr Sulaiman Al Habib Medical Group, Riyadh, Kingdom of Saudi Arabia.
Abstract
Importance: Helmet noninvasive ventilation has been used in patients with COVID-19 with the premise that helmet interface is more effective than mask interface in delivering prolonged treatments with high positive airway pressure, but data about its effectiveness are limited.
Objective: To evaluate whether helmet noninvasive ventilation compared with usual respiratory support reduces mortality in patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
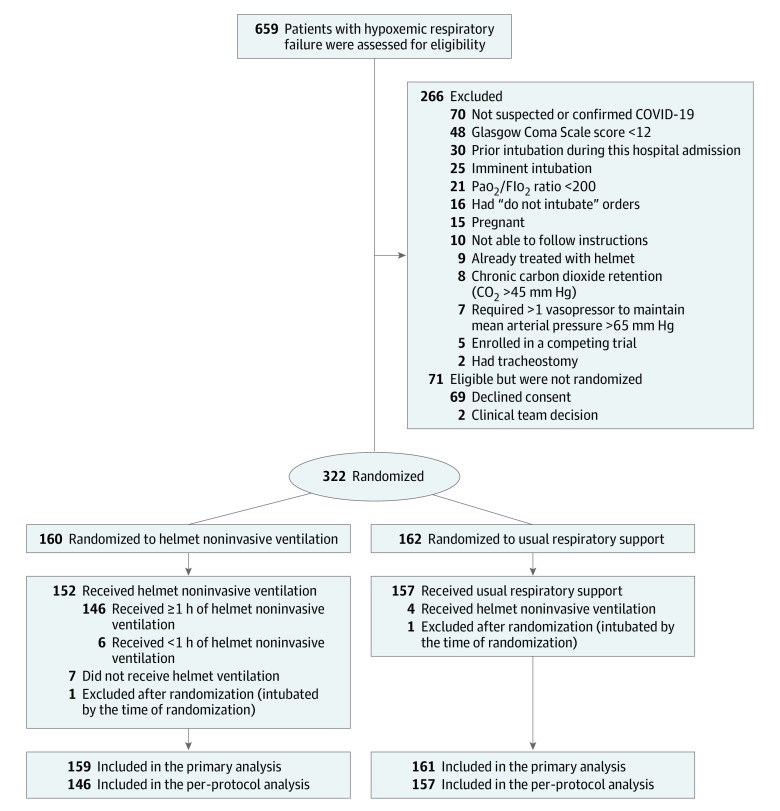
Design, setting, and participants: This was a multicenter, pragmatic, randomized clinical trial that was conducted in 8 sites in Saudi Arabia and Kuwait between February 8, 2021, and November 16, 2021. Adult patients with acute hypoxemic respiratory failure (n = 320) due to suspected or confirmed COVID-19 were included. The final follow-up date for the primary outcome was December 14, 2021.
Interventions: Patients were randomized to receive helmet noninvasive ventilation (n = 159) or usual respiratory support (n = 161), which included mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen.
Main outcomes and measures: The primary outcome was 28-day all-cause mortality. There were 12 prespecified secondary outcomes, including endotracheal intubation, barotrauma, skin pressure injury, and serious adverse events.
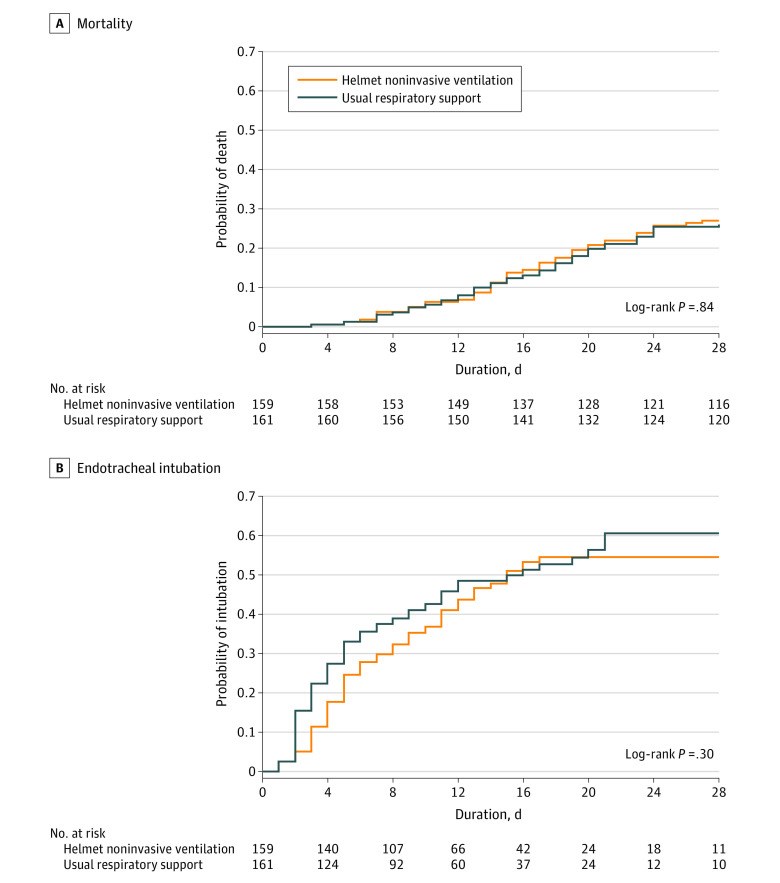
Results: Among 322 patients who were randomized, 320 were included in the primary analysis, all of whom completed the trial. Median age was 58 years, and 187 were men (58.4%). Within 28 days, 43 of 159 patients (27.0%) died in the helmet noninvasive ventilation group compared with 42 of 161 (26.1%) in the usual respiratory support group (risk difference, 1.0% [95% CI, -8.7% to 10.6%]; relative risk, 1.04 [95% CI, 0.72-1.49]; P = .85). Within 28 days, 75 of 159 patients (47.2%) required endotracheal intubation in the helmet noninvasive ventilation group compared with 81 of 161 (50.3%) in the usual respiratory support group (risk difference, -3.1% [95% CI, -14.1% to 7.8%]; relative risk, 0.94 [95% CI, 0.75-1.17]). There were no significant differences between the 2 groups in any of the prespecified secondary end points. Barotrauma occurred in 30 of 159 patients (18.9%) in the helmet noninvasive ventilation group and 25 of 161 (15.5%) in the usual respiratory support group. Skin pressure injury occurred in 5 of 159 patients (3.1%) in the helmet noninvasive ventilation group and 10 of 161 (6.2%) in the usual respiratory support group. There were 2 serious adverse events in the helmet noninvasive ventilation group and 1 in the usual respiratory support group.
Conclusions and relevance: Results of this study suggest that helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia. However, interpretation of the findings is limited by imprecision in the effect estimate, which does not exclude potentially clinically important benefit or harm.
Trial registration: ClinicalTrials.gov Identifier: NCT04477668.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Al-Dorzi reported receiving honoraria for educational activities from Sanofi outside the submitted work. No other disclosures were reported.
Figures
Similar articles
Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, Montomoli J, Falò G, Tonetti T, Cutuli SL, Pintaudi G, Tanzarella ES, Piervincenzi E, Bongiovanni F, Dell'Anna AM, Delle Cese L, Berardi C, Carelli S, Bocci MG, Montini L, Bello G, Natalini D, De Pascale G, Velardo M, Volta CA, Ranieri VM, Conti G, Maggiore SM, Antonelli M; COVID-ICU Gemelli Study Group.JAMA. 2021 May 4;325(17):1731-1743. doi: 10.1001/jama.2021.4682.PMID: 33764378 Free PMC article. Clinical Trial.
Jaber S, Lescot T, Futier E, Paugam-Burtz C, Seguin P, Ferrandiere M, Lasocki S, Mimoz O, Hengy B, Sannini A, Pottecher J, Abback PS, Riu B, Belafia F, Constantin JM, Masseret E, Beaussier M, Verzilli D, De Jong A, Chanques G, Brochard L, Molinari N; NIVAS Study Group.JAMA. 2016 Apr 5;315(13):1345-53. doi: 10.1001/jama.2016.2706.PMID: 26975890 Clinical Trial.
Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, Bradley JM, Dark P, Dave C, De Soyza A, Dennis AV, Devrell A, Fairbairn S, Ghani H, Gorman EA, Green CA, Hart N, Hee SW, Kimbley Z, Madathil S, McGowan N, Messer B, Naisbitt J, Norman C, Parekh D, Parkin EM, Patel J, Regan SE, Ross C, Rostron AJ, Saim M, Simonds AK, Skilton E, Stallard N, Steiner M, Vancheeswaran R, Yeung J, McAuley DF; RECOVERY-RS Collaborators.JAMA. 2022 Feb 8;327(6):546-558. doi: 10.1001/jama.2022.0028.PMID: 35072713 Free PMC article. Clinical Trial.
Xu XP, Zhang XC, Hu SL, Xu JY, Xie JF, Liu SQ, Liu L, Huang YZ, Guo FM, Yang Y, Qiu HB.Crit Care Med. 2017 Jul;45(7):e727-e733. doi: 10.1097/CCM.0000000000002361.PMID: 28441237 Free PMC article. Review.
Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, Barbas CSV, de Acilu MG, Cutuli SL, Bongiovanni F, Amato M, Frat JP, Mauri T, Kress JP, Mancebo J, Antonelli M.Intensive Care Med. 2021 Aug;47(8):851-866. doi: 10.1007/s00134-021-06459-2. Epub 2021 Jul 7.PMID: 34232336 Free PMC article. Review.
Cited by
The COVID-19 Driving Force: How It Shaped the Evidence of Non-Invasive Respiratory Support.
Jalil Y, Ferioli M, Dres M.J Clin Med. 2023 May 16;12(10):3486. doi: 10.3390/jcm12103486.PMID: 37240592 Free PMC article. Review.
Personalized noninvasive respiratory support for acute hypoxemic respiratory failure.
Grieco DL, Munshi L, Piquilloud L.Intensive Care Med. 2023 Apr 28:1-4. doi: 10.1007/s00134-023-07048-1. Online ahead of print.PMID: 37115260 Free PMC article. No abstract available.
Helmet trials: resolving the puzzle.
Arabi YM, Patel BK, Antonelli M.Intensive Care Med. 2023 Apr;49(4):458-461. doi: 10.1007/s00134-023-07004-z. Epub 2023 Mar 1.PMID: 36856774 Free PMC article. No abstract available.
Kasarabada A, Barker K, Ganoe T, Clevenger L, Visco C, Gibson J, Karimi R, Naderi N, Lam B, Stepanova M, Henry L, King C, Desai M.PLoS One. 2023 Feb 16;18(2):e0281859. doi: 10.1371/journal.pone.0281859. eCollection 2023.PMID: 36795723 Free PMC article.
Fiedler MO, Dietrich M, Reuß CJ, Bernhard M, Beynon C, Hecker A, Jungk C, Nusshag C, Michalski D, Weigand MA, Brenner T.Anaesthesiologie. 2023 Mar;72(3):199-208. doi: 10.1007/s00101-023-01250-y. Epub 2023 Jan 25.PMID: 36695839 Free PMC article. German. No abstract available.