Semiquantitative Decision Tools for FMD Emergency Vaccination Informed by Field Observations and Simulated Outbreak Data
Affiliations
Affiliations
- 1Department of Diagnostic and Scientific Advice, National Veterinary Institute, Technical University of Denmark , Copenhagen , Denmark.
- 2Environment and Life Sciences Research Center, Kuwait Institute for Scientific Research, Kuwait City, Kuwait; Department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, St. Paul, USA.
- 3Department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota , St. Paul , USA.
Abstract
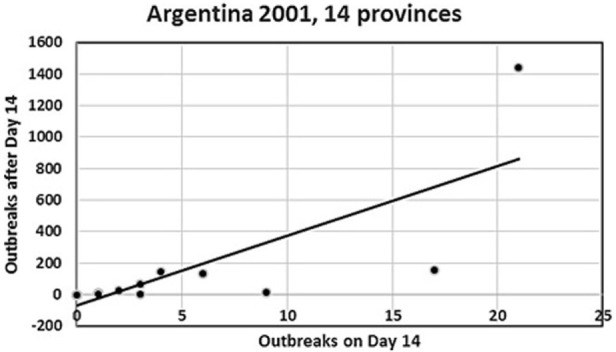
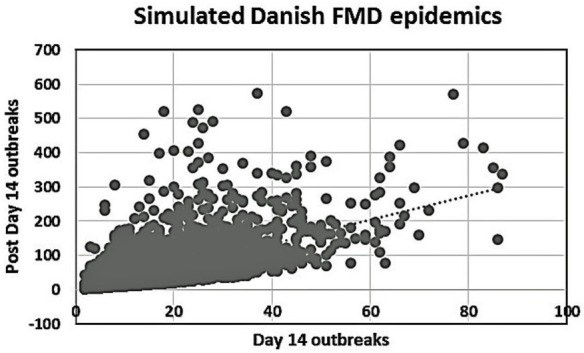
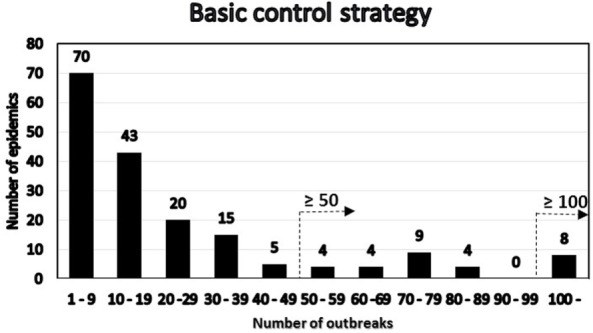
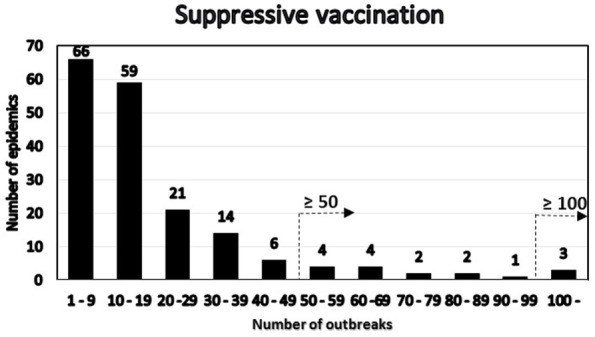
We present two simple, semiquantitative model-based decision tools, based on the principle of first 14 days incidence (FFI). The aim is to estimate the likelihood and the consequences, respectively, of the ultimate size of an ongoing FMD epidemic. The tools allow risk assessors to communicate timely, objectively, and efficiently to risk managers and less technically inclined stakeholders about the potential of introducing FMD suppressive emergency vaccination. To explore the FFI principle with complementary field data, we analyzed the FMD outbreaks in Argentina in 2001, with the 17 affected provinces as the units of observation. Two different vaccination strategies were applied during this extended epidemic. In a series of 5,000 Danish simulated FMD epidemics, the numbers of outbreak herds at day 14 and at the end of the epidemics were estimated under different control strategies. To simplify and optimize the presentation of the resulting data for urgent decisions to be made by the risk managers, we estimated the sensitivity, specificity, as well as the negative and positive predictive values, using a chosen day-14 outbreak number as predictor of the magnitude of the number of remaining post-day-14 outbreaks under a continued basic control strategy. Furthermore, during an ongoing outbreak, the actual cumulative number of detected infected herds at day 14 will be known exactly. Among the number of epidemics lasting >14 days out of the 5,000 simulations under the basic control scenario, we selected those with an assumed accumulated number of detected outbreaks at day 14. The distribution of the estimated number of detected outbreaks at the end of the simulated epidemics minus the number at day 14 was estimated for the epidemics lasting more than 14 days. For comparison, the same was done for identical epidemics (i.e., seeded with the same primary outbreak herds) under a suppressive vaccination scenario. The results indicate that, during the course of an FMD epidemic, simulated likelihood predictions of the remaining epidemic size and of potential benefits of alternative control strategies can be presented to risk managers and other stakeholders in objective and easily communicable ways.
Keywords: Foot-and-Mouth Disease; disease control; epidemics; modeling; risk communication.
Figures
Similar articles
Boklund A, Halasa T, Christiansen LE, Enøe C.Prev Vet Med. 2013 Sep 1;111(3-4):206-19. doi: 10.1016/j.prevetmed.2013.05.008. Epub 2013 Jun 20.PMID: 23791121
Decisions on control of foot-and-mouth disease informed using model predictions.
Halasa T, Willeberg P, Christiansen LE, Boklund A, Alkhamis M, Perez A, Enøe C.Prev Vet Med. 2013 Nov 1;112(3-4):194-202. doi: 10.1016/j.prevetmed.2013.09.003. Epub 2013 Sep 12.PMID: 24080392
Sanson RL, Yu ZD, Rawdon TG, van Andel M.N Z Vet J. 2021 Nov;69(6):313-326. doi: 10.1080/00480169.2021.1921069. Epub 2021 Jun 9.PMID: 33886430
Foot-and-Mouth disease control using vaccination: the Dutch experience in 2001.
Pluimers FH.Dev Biol (Basel). 2004;119:41-9.PMID: 15742617 Review.
Review: Evaluation of Foot-and-Mouth Disease Control Using Fault Tree Analysis.
Isoda N, Kadohira M, Sekiguchi S, Schuppers M, Stärk KD.Transbound Emerg Dis. 2015 Jun;62(3):233-44. doi: 10.1111/tbed.12116. Epub 2013 Jun 28.PMID: 23809890 Review.
Cited by
FMD vaccine allocation and surveillance resourcing options for a potential Australian incursion.
Seitzinger AH, Garner MG, Bradhurst R, Roche S, Breed AC, Capon T, Miller C, Tapsuwan S.Aust Vet J. 2022 Nov;100(11):550-561. doi: 10.1111/avj.13195. Epub 2022 Sep 15.PMID: 36106431 Free PMC article.
Hafi A, Addai D, Breed AC, Bradhurst R, Capon T, Garner MG, Miller C, Pinol J, Seitzinger AH, Tapsuwan S.Aust Vet J. 2022 Apr;100(4):150-161. doi: 10.1111/avj.13141. Epub 2022 Jan 20.PMID: 35049045 Free PMC article.
Evaluating vaccination strategies to control foot-and-mouth disease: a country comparison study.
Rawdon TG, Garner MG, Sanson RL, Stevenson MA, Cook C, Birch C, Roche SE, Patyk KA, Forde-Folle KN, Dubé C, Smylie T, Yu ZD.Epidemiol Infect. 2018 Jul;146(9):1138-1150. doi: 10.1017/S0950268818001243. Epub 2018 May 22.PMID: 29785893 Free PMC article.
Control fast or control smart: When should invading pathogens be controlled?
Thompson RN, Gilligan CA, Cunniffe NJ.PLoS Comput Biol. 2018 Feb 16;14(2):e1006014. doi: 10.1371/journal.pcbi.1006014. eCollection 2018 Feb.PMID: 29451878 Free PMC article.
Resource Estimations in Contingency Planning for Foot-and-Mouth Disease.
Boklund A, Mortensen S, Johansen MH, Halasa T.Front Vet Sci. 2017 May 11;4:64. doi: 10.3389/fvets.2017.00064. eCollection 2017.PMID: 28553640 Free PMC article.
KMEL References
References
-
- Council of the European Union. Council Directive 2003/85/EC on Community measures for the control of foot-and-mouth disease. Off J Eur U (2003) 306:1–87.
-
- Willeberg P. Simple decisions tools informed by model predictions when considering FMD emergency vaccination strategies. Open Session of the EuFMD Standing Technical Committee. Jerez, Spain: (2012). p. 29–31. Available from: http://www.fao.org/fileadmin/user_upload/eufmd/docs/Open_Session2012/App...
-
- Willeberg P, Halasa T, Alkhamis M, Boklund A, Perez A, Enøe C. Decisions on FMD emergency vaccination informed by outbreak data and model predictions. Book of Abstracts, 13th International Symposium on Veterinary Epidemiology and Economics (ISVEE). Wageningen, NL: Wageningen Academic Publishers; (2012). p. 385.
-
- Halasa T, Willeberg P, Christiansen LE, Boklund A, AlKhamis M, Perez A, et al. Decisions on foot-and-mouth disease control informed by model prediction. Proceedings, Society for Veterinary Epidemiology and Preventive Medicine (SVEPM), Annual Meeting. Madrid: (2013). p. 13–22.
-
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput (2000) 10:325–37.10.1023/A:1008929526011 - DOI
-
- Sanson RL, Stevenson MA, Moles-Benfell N. Quantifying local spread probabilities for foot-and-mouth disease. Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics (ISVEE) Cairns, Australia (2006).
-
- World Organisation for Animal Health (OIE). Handbook on Import Risk Analysis for Animals and Animal Products. 2nd ed (Vol. 1) Paris: OIE; (2010).
-
- European Commission for the Control of Foot-and-Mouth Disease. 39th General Session, General Session, Recommendation 6. Rome: FAO; (2011). Available from: http://www.fao.org/fileadmin/user_upload/eufmd/docs/39th_Gen_session/Fin...