Effect of Disease-Modifying Therapy on Disability in Relapsing-Remitting Multiple Sclerosis Over 15 Years
Tomas Kalincik 1, Ibrahima Diouf 2, Sifat Sharmin 2, Charles Malpas 2, Tim Spelman 2, Dana Horakova 2, Eva Kubala Havrdova 2, Maria Trojano 2, Guillermo Izquierdo 2, Alessandra Lugaresi 2, Alexandre Prat 2, Marc Girard 2, Pierre Duquette 2, Pierre Grammond 2, Vilija Jokubaitis 2, Anneke van der Walt 2, Francois Grand'Maison 2, Patrizia Sola 2, Diana Ferraro 2, Vahid Shaygannejad 2, Raed Alroughani 2, Raymond Hupperts 2, Murat Terzi 2, Cavit Boz 2, Jeannette Lechner-Scott 2, Eugenio Pucci 2, Vincent Van Pesch 2, Franco Granella 2, Roberto Bergamaschi 2, Daniele Spitaleri 2, Mark Slee 2, Steve Vucic 2, Radek Ampapa 2, Pamela McCombe 2, Cristina Ramo-Tello 2, Julie Prevost 2, Javier Olascoaga 2, Edgardo Cristiano 2, Michael Barnett 2, Maria Laura Saladino 2, Jose Luis Sanchez-Menoyo 2, Suzanne Hodgkinson 2, Csilla Rozsa 2, Stella Hughes 2, Fraser Moore 2, Cameron Shaw 2, Ernest Butler 2, Olga Skibina 2, Orla Gray 2, Allan Kermode 2, Tunde Csepany 2, Bhim Singhal 2, Neil Shuey 2, Imre Piroska 2, Bruce Taylor 2, Magdolna Simo 2, Carmen-Adella Sirbu 2, Attila Sas 2, Helmut Butzkueven 2; MSBase Study Group
Affiliations
Affiliations
- From CORe (T.K., I.D., S.S., C.M.), Department of Medicine, University of Melbourne; MS Centre (T.K., I.D., S.S., C.M.), Department of Neurology, Royal Melbourne Hospital, Australia; Karolinska Institute (T.S.), Stockholm, Sweden; Department of Neuroscience (T.S., V.J., A.v.d.W., O.S., H.B.), Central Clinical School, Monash University, Melbourne; Burnet Institute (T.S.), Melbourne, Australia; Department of Neurology and Center of Clinical Neuroscience (D.H., E.K.H.), General University Hospital and Charles University in Prague, Czech Republic; Department of Basic Medical Sciences, Neuroscience and Sense Organs (M. Trojano), University of Bari, Italy; Hospital Universitario Virgen Macarena (G.I.), Sevilla, Spain; Department of Neuroscience, Imaging and Clinical Sciences (A.L.), University "G. d'Annunzio," Chieti; Department of Biomedical and Neuromotor Sciences (A.L.), University of Bologna, IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy; Hopital Notre Dame (A.P., M.G., P.D.), Montreal; CHUM and Universite de Montreal (A.P., M.G., P.D.); CISSS Chaudière-Appalache (P.G.), Levis, Canada; Department of Neurology (V.J., A.v.d.W., O.S., H.B.), Alfred Hospital, Melbourne, Australia; Neuro Rive-Sud (F. Grand'Maison), Quebec, Canada; Department of Neuroscience (P.S., D.F.), Azienda Ospedaliera Universitaria, Modena, Italy; Isfahan University of Medical Sciences (V.S.), Isfahan, Iran; Amiri Hospital (R. Alroughani), Kuwait City, Kuwait; Zuyderland Ziekenhuis (R.H.), Sittard, the Netherlands; Medical Faculty (M. Terzi), 19 Mayis University, Samsun; KTU Medical Faculty Farabi Hospital (C.B.), Karadeniz Technical University, Trabzon, Turkey; School of Medicine and Public Health (J.L.-S.), University Newcastle; Department of Neurology (J.L.-S.), John Hunter Hospital, Newcastle, Australia; UOC Neurologia (E.P.), Azienda Sanitaria Unica Regionale Marche-AV3, Macerata, Italy; Cliniques Universitaires Saint-Luc (V.V.P.), Brussels, Belgium; University of Parma (F. Granella); C. Mondino National Neurological Institute (R.B.), Pavia; Azienda Ospedaliera di Rilievo Nazionale San Giuseppe Moscati Avellino (D.S.), Italy; Flinders University (M. Slee), Adelaide; Westmead Hospital (S.V.), Sydney, Australia; Nemocnice Jihlava (R. Ampapa), Czech Republic; University of Queensland (P.M.), Brisbane; Royal Brisbane and Women's Hospital (P.M.), Brisbane, Australia; Hospital Germans Trias i Pujol (C.R.-T.), Badalona, Spain; CSSS Saint-Jérôme (J.P.), Canada; Hospital Universitario Donostia (J.O.), Paseo de Begiristain, San Sebastián, Spain; Hospital Italiano (E.C.), Buenos Aires, Argentina; Brain and Mind Centre (M.B.), University of Sydney, Australia; INEBA-Institute of Neuroscience Buenos Aires (M.L.S.), Argentina; Hospital de Galdakao-Usansolo (J.L.S.-M.), Galdakao, Spain; Liverpool Hospital (S. Hodgkinson), Sydney, Australia; Jahn Ferenc Teaching Hospital (C.R.), Budapest, Hungary; Craigavon Area Hospital (S. Hughes), UK; Jewish General Hospital (F.M.), Montreal, Canada; Deakin University (C.S.), Geelong; Monash Medical Centre (E.B.), Melbourne, Australia; South East Trust (O.G.), Belfast, UK; Perron Institute (A.K.), University of Western Australia, Nedlands; Institute of Immunology and Infectious Diseases (A.K.), Murdoch University; Sir Charles Gairdner Hospital (A.K.), Perth, Australia; Department of Neurology (T.C.), Faculty of Medicine, University of Debrecen, Hungary; Bombay Hospital Institute of Medical Sciences (B.S.), Mumbai, India; St Vincents Hospital (N.S.), Fitzroy, Melbourne, Australia; Veszprém Megyei Csolnoky Ferenc Kórház zrt (I.P.), Veszprem, Hungary; Royal Hobart Hospital (B.T.), Australia; Semmelweis University Budapest (M. Simo), Hungary; Central Military Emergency University Hospital (C.-A.S.), Bucharest; Titu Maiorescu University (C.-A.S.), Bucharest, Romania; BAZ County Hospital (A.S.), Miskolc, Hungary; and Box Hill Hospital (H.B.), Melbourne, Australia. tomas.kalincik@unimelb.edu.
- 2From CORe (T.K., I.D., S.S., C.M.), Department of Medicine, University of Melbourne; MS Centre (T.K., I.D., S.S., C.M.), Department of Neurology, Royal Melbourne Hospital, Australia; Karolinska Institute (T.S.), Stockholm, Sweden; Department of Neuroscience (T.S., V.J., A.v.d.W., O.S., H.B.), Central Clinical School, Monash University, Melbourne; Burnet Institute (T.S.), Melbourne, Australia; Department of Neurology and Center of Clinical Neuroscience (D.H., E.K.H.), General University Hospital and Charles University in Prague, Czech Republic; Department of Basic Medical Sciences, Neuroscience and Sense Organs (M. Trojano), University of Bari, Italy; Hospital Universitario Virgen Macarena (G.I.), Sevilla, Spain; Department of Neuroscience, Imaging and Clinical Sciences (A.L.), University "G. d'Annunzio," Chieti; Department of Biomedical and Neuromotor Sciences (A.L.), University of Bologna, IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy; Hopital Notre Dame (A.P., M.G., P.D.), Montreal; CHUM and Universite de Montreal (A.P., M.G., P.D.); CISSS Chaudière-Appalache (P.G.), Levis, Canada; Department of Neurology (V.J., A.v.d.W., O.S., H.B.), Alfred Hospital, Melbourne, Australia; Neuro Rive-Sud (F. Grand'Maison), Quebec, Canada; Department of Neuroscience (P.S., D.F.), Azienda Ospedaliera Universitaria, Modena, Italy; Isfahan University of Medical Sciences (V.S.), Isfahan, Iran; Amiri Hospital (R. Alroughani), Kuwait City, Kuwait; Zuyderland Ziekenhuis (R.H.), Sittard, the Netherlands; Medical Faculty (M. Terzi), 19 Mayis University, Samsun; KTU Medical Faculty Farabi Hospital (C.B.), Karadeniz Technical University, Trabzon, Turkey; School of Medicine and Public Health (J.L.-S.), University Newcastle; Department of Neurology (J.L.-S.), John Hunter Hospital, Newcastle, Australia; UOC Neurologia (E.P.), Azienda Sanitaria Unica Regionale Marche-AV3, Macerata, Italy; Cliniques Universitaires Saint-Luc (V.V.P.), Brussels, Belgium; University of Parma (F. Granella); C. Mondino National Neurological Institute (R.B.), Pavia; Azienda Ospedaliera di Rilievo Nazionale San Giuseppe Moscati Avellino (D.S.), Italy; Flinders University (M. Slee), Adelaide; Westmead Hospital (S.V.), Sydney, Australia; Nemocnice Jihlava (R. Ampapa), Czech Republic; University of Queensland (P.M.), Brisbane; Royal Brisbane and Women's Hospital (P.M.), Brisbane, Australia; Hospital Germans Trias i Pujol (C.R.-T.), Badalona, Spain; CSSS Saint-Jérôme (J.P.), Canada; Hospital Universitario Donostia (J.O.), Paseo de Begiristain, San Sebastián, Spain; Hospital Italiano (E.C.), Buenos Aires, Argentina; Brain and Mind Centre (M.B.), University of Sydney, Australia; INEBA-Institute of Neuroscience Buenos Aires (M.L.S.), Argentina; Hospital de Galdakao-Usansolo (J.L.S.-M.), Galdakao, Spain; Liverpool Hospital (S. Hodgkinson), Sydney, Australia; Jahn Ferenc Teaching Hospital (C.R.), Budapest, Hungary; Craigavon Area Hospital (S. Hughes), UK; Jewish General Hospital (F.M.), Montreal, Canada; Deakin University (C.S.), Geelong; Monash Medical Centre (E.B.), Melbourne, Australia; South East Trust (O.G.), Belfast, UK; Perron Institute (A.K.), University of Western Australia, Nedlands; Institute of Immunology and Infectious Diseases (A.K.), Murdoch University; Sir Charles Gairdner Hospital (A.K.), Perth, Australia; Department of Neurology (T.C.), Faculty of Medicine, University of Debrecen, Hungary; Bombay Hospital Institute of Medical Sciences (B.S.), Mumbai, India; St Vincents Hospital (N.S.), Fitzroy, Melbourne, Australia; Veszprém Megyei Csolnoky Ferenc Kórház zrt (I.P.), Veszprem, Hungary; Royal Hobart Hospital (B.T.), Australia; Semmelweis University Budapest (M. Simo), Hungary; Central Military Emergency University Hospital (C.-A.S.), Bucharest; Titu Maiorescu University (C.-A.S.), Bucharest, Romania; BAZ County Hospital (A.S.), Miskolc, Hungary; and Box Hill Hospital (H.B.), Melbourne, Australia.
Abstract
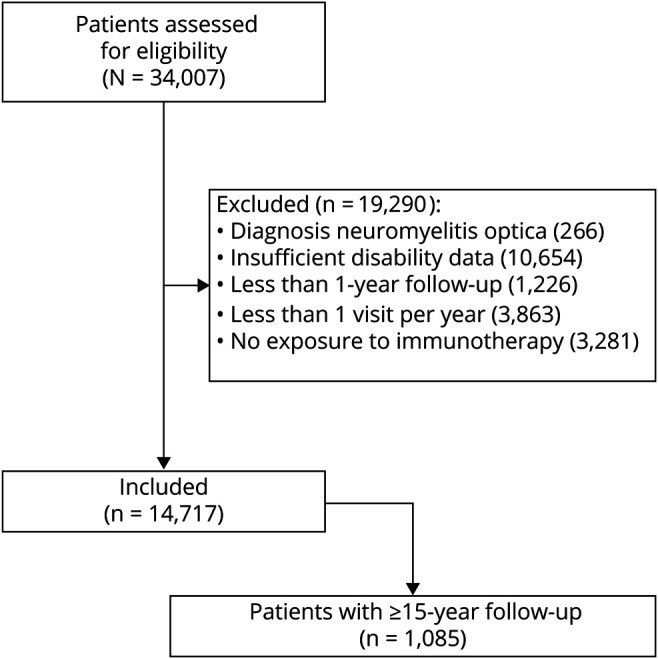
Objective: To test the hypothesis that immunotherapy prevents long-term disability in relapsing-remitting multiple sclerosis (MS), we modeled disability outcomes in 14,717 patients.
Methods: We studied patients from MSBase followed for ≥1 year, with ≥3 visits, ≥1 visit per year, and exposed to MS therapy, and a subset of patients with ≥15-year follow-up. Marginal structural models were used to compare the cumulative hazards of 12-month confirmed increase and decrease in disability, Expanded Disability Status Scale (EDSS) step 6, and the incidence of relapses between treated and untreated periods. Marginal structural models were continuously readjusted for patient age, sex, pregnancy, date, disease course, time from first symptom, prior relapse history, disability, and MRI activity.
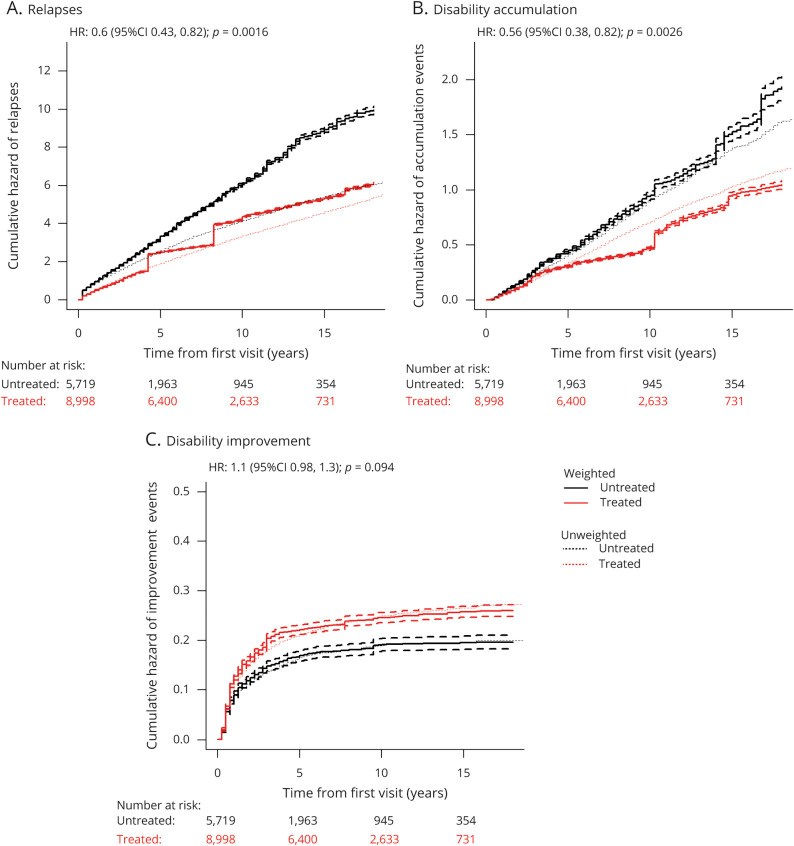
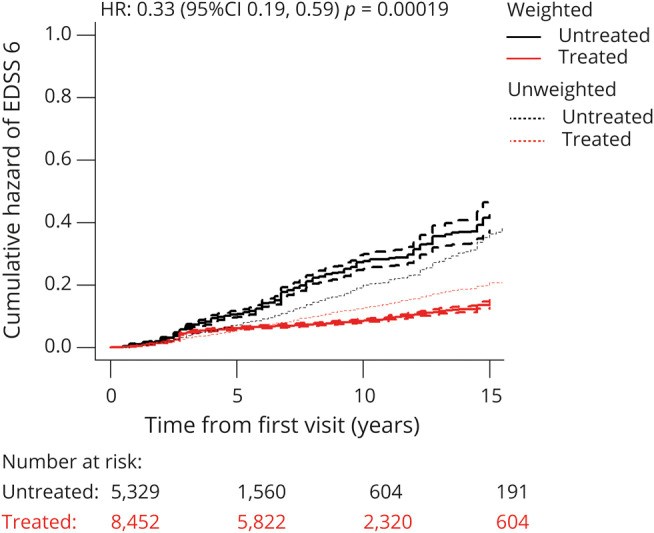
Results: A total of 14,717 patients were studied. During the treated periods, patients were less likely to experience relapses (hazard ratio 0.60, 95% confidence interval [CI] 0.43-0.82, p = 0.0016), worsening of disability (0.56, 0.38-0.82, p = 0.0026), and progress to EDSS step 6 (0.33, 0.19-0.59, p = 0.00019). Among 1,085 patients with ≥15-year follow-up, the treated patients were less likely to experience relapses (0.59, 0.50-0.70, p = 10-9) and worsening of disability (0.81, 0.67-0.99, p = 0.043).
Conclusion: Continued treatment with MS immunotherapies reduces disability accrual by 19%-44% (95% CI 1%-62%), the risk of need of a walking aid by 67% (95% CI 41%-81%), and the frequency of relapses by 40-41% (95% CI 18%-57%) over 15 years. This study provides evidence that disease-modifying therapies are effective in improving disability outcomes in relapsing-remitting MS over the long term.
Classification of evidence: This study provides Class IV evidence that, for patients with relapsing-remitting MS, long-term exposure to immunotherapy prevents neurologic disability.
Figures
Similar articles
Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, Wilkins A, Pearson O, Ziemssen T, Hutchinson M, McGuigan C, Jokubaitis V, Spelman T, Horakova D, Havrdova E, Trojano M, Izquierdo G, Lugaresi A, Prat A, Girard M, Duquette P, Grammond P, Alroughani R, Pucci E, Sola P, Hupperts R, Lechner-Scott J, Terzi M, Van Pesch V, Rozsa C, Grand'Maison F, Boz C, Granella F, Slee M, Spitaleri D, Olascoaga J, Bergamaschi R, Verheul F, Vucic S, McCombe P, Hodgkinson S, Sanchez-Menoyo JL, Ampapa R, Simo M, Csepany T, Ramo C, Cristiano E, Barnett M, Butzkueven H, Coles A; MSBase Study Group.Lancet Neurol. 2017 Apr;16(4):271-281. doi: 10.1016/S1474-4422(17)30007-8. Epub 2017 Feb 11.PMID: 28209331
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, Patti F, Lugaresi A, Zimatore GB, Marrosu MG, Amato MP, Bertolotto A, Bergamaschi R, Granella F, Coniglio G, Tedeschi G, Sola P, Lus G, Ferrò MT, Iuliano G, Corea F, Protti A, Cavalla P, Guareschi A, Rodegher M, Paolicelli D, Tortorella C, Lepore V, Prosperini L, Saccà F, Baroncini D, Comi G, Trojano M; Italian iMed-Web database.Brain. 2015 Nov;138(Pt 11):3275-86. doi: 10.1093/brain/awv260. Epub 2015 Sep 11.PMID: 26362907
Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, Girard M, Duquette P, Trojano M, Lugaresi A, Bergamaschi R, Grammond P, Alroughani R, Hupperts R, McCombe P, Van Pesch V, Sola P, Ferraro D, Grand'Maison F, Terzi M, Lechner-Scott J, Flechter S, Slee M, Shaygannejad V, Pucci E, Granella F, Jokubaitis V, Willis M, Rice C, Scolding N, Wilkins A, Pearson OR, Ziemssen T, Hutchinson M, Harding K, Jones J, McGuigan C, Butzkueven H, Kalincik T, Robertson N; MSBase Study Group.JAMA. 2019 Jan 15;321(2):175-187. doi: 10.1001/jama.2018.20588.PMID: 30644981 Free PMC article.
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.
Filippini G, Del Giovane C, Vacchi L, D'Amico R, Di Pietrantonj C, Beecher D, Salanti G.Cochrane Database Syst Rev. 2013 Jun 6;(6):CD008933. doi: 10.1002/14651858.CD008933.pub2.PMID: 23744561 Review.
Teriflunomide for multiple sclerosis.
He D, Zhang C, Zhao X, Zhang Y, Dai Q, Li Y, Chu L.Cochrane Database Syst Rev. 2016 Mar 22;3:CD009882. doi: 10.1002/14651858.CD009882.pub3.PMID: 27003123 Review.
Cited by
Multiple Sclerosis and Autoimmune Comorbidities.
Nociti V, Romozzi M.J Pers Med. 2022 Nov 3;12(11):1828. doi: 10.3390/jpm12111828.PMID: 36579555 Free PMC article. Review.
Excess costs of multiple sclerosis: a register-based study in Sweden.
Murley C, Tinghög P, Teni FS, Machado A, Alexanderson K, Hillert J, Karampampa K, Friberg E.Eur J Health Econ. 2022 Nov 23:1-15. doi: 10.1007/s10198-022-01547-6. Online ahead of print.PMID: 36418785 Free PMC article.
Ford CC, Cohen JA, Goodman AD, Lindsey JW, Lisak RP, Luzzio C, Pruitt A, Rose J, Rus H, Wolinsky JS, Kadosh SE, Bernstein-Hanlon E, Stark Y, Alexander JK.Mult Scler. 2022 Oct;28(11):1729-1743. doi: 10.1177/13524585221094239. Epub 2022 Jun 29.PMID: 35768939 Free PMC article. Clinical Trial.
Brill L, Rechtman A, Shifrin A, Rozenberg A, Afanasiev S, Zveik O, Haham N, Levin N, Vaknin-Dembinsky A.Mult Scler Relat Disord. 2022 Jul;63:103863. doi: 10.1016/j.msard.2022.103863. Epub 2022 May 10.PMID: 35667316 Free PMC article.
Lefort M, Sharmin S, Andersen JB, Vukusic S, Casey R, Debouverie M, Edan G, Ciron J, Ruet A, De Sèze J, Maillart E, Zephir H, Labauge P, Defer G, Lebrun-Frenay C, Moreau T, Berger E, Clavelou P, Pelletier J, Stankoff B, Gout O, Thouvenot E, Heinzlef O, Al-Khedr A, Bourre B, Casez O, Cabre P, Montcuquet A, Wahab A, Camdessanché JP, Maurousset A, Ben Nasr H, Hankiewicz K, Pottier C, Maubeuge N, Dimitri-Boulos D, Nifle C, Laplaud DA, Horakova D, Havrdova EK, Alroughani R, Izquierdo G, Eichau S, Ozakbas S, Patti F, Onofrj M, Lugaresi A, Terzi M, Grammond P, Grand'Maison F, Yamout B, Prat A, Girard M, Duquette P, Boz C, Trojano M, McCombe P, Slee M, Lechner-Scott J, Turkoglu R, Sola P, Ferraro D, Granella F, Shaygannejad V, Prevost J, Maimone D, Skibina O, Buzzard K, Van der Walt A, Karabudak R, Van Wijmeersch B, Csepany T, Spitaleri D, Vucic S, Koch-Henriksen N, Sellebjerg F, Soerensen PS, Hilt Christensen CC, Rasmussen PV, Jensen MB, Frederiksen JL, Bramow S, Mathiesen HK, Schreiber KI, Butzkueven H, Magyari M, Kalincik T, Leray E.BMC Med Res Methodol. 2022 May 30;22(1):155. doi: 10.1186/s12874-022-01623-8.PMID: 35637426 Free PMC article.