Helmet noninvasive ventilation for COVID-19 patients (Helmet-COVID): statistical analysis plan for a randomized controlled trial
Yaseen Arabi 1, Sara Aldekhyl 2, Saad Al Qahtani 3, Hasan M Al-Dorzi 3, Sheryl Ann Abdukahil 3, Jesna Jose 4, Mohammad Khulaif Al Harbi 5, Husain Al Haji 6, Mohammed Al Mutairi 6, Omar Al Zumai 6, Eman Al Qasim 7, Wedyan Al Wehaibi 3, Mohammed Alshahrani 8, Talal Albrahim 9, Ahmed Mady 10 11, Ali Al Bshabshe 12, Zohair Al Aseri 13, Zainab Al Duhailib 14, Ayman Kharaba 15, Rakan Alqahtani 16, Haifa Algethamy 17, Omar Alfaris 6, Omar Alnafel 18, Abdulrahman A Al-Fares 19, Haytham Tlayjeh 3
Affiliations
Affiliations
- 1Intensive Care Department, Ministry of National Guard Health Affairs, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia. yaseenarabi@yahoo.com.
- 2College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia.
- 3Intensive Care Department, Ministry of National Guard Health Affairs, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
- 4Bioinformatics and Biostatistics Department, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia.
- 5Department of Anesthesia, Ministry of National Guard Health Affairs, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
- 6Respiratory Services Department, Ministry of National Guard Health Affairs, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
- 7Research Office, King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia.
- 8Department of Emergency and Critical Care, King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Al Khobar, Kingdom of Saudi Arabia.
- 9Department of Critical Care, King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Al Khobar, Kingdom of Saudi Arabia.
- 10Intensive Care Department, King Saud Medical City, Riyadh, Saudi Arabia.
- 11College of Medicine, Tanta University, Tanta, Egypt.
- 12Department of Critical Care Medicine, King Khalid University, Aseer Central Hospital, Abha, Kingdom of Saudi Arabia.
- 13Emergency and Intensive Care Departments, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
- 14Adult Critical Care Department, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia.
- 15Pulmonary & Critical Care Departments, King Fahad Hospital Madinah Critical Care Units, Madinah, Saudi Arabia.
- 16Department of Critical Care, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
- 17Department of Anesthesia and Critical Care, King Abdulaziz University, King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
- 18Internal Medicine and Intensive Care Department, King Salman Specialist Hospital, Hail, Saudi Arabia.
- 19Department of Anesthesia, Critical Care Medicine and Pain Medicine, Al-Amiri Hospital, Ministry of Health, Kuwait, Kuwait.
Abstract
Background: Noninvasive respiratory support is frequently needed for patients with acute hypoxemic respiratory failure due to coronavirus disease 19 (COVID-19). Helmet noninvasive ventilation has multiple advantages over other oxygen support modalities but data about effectiveness are limited.
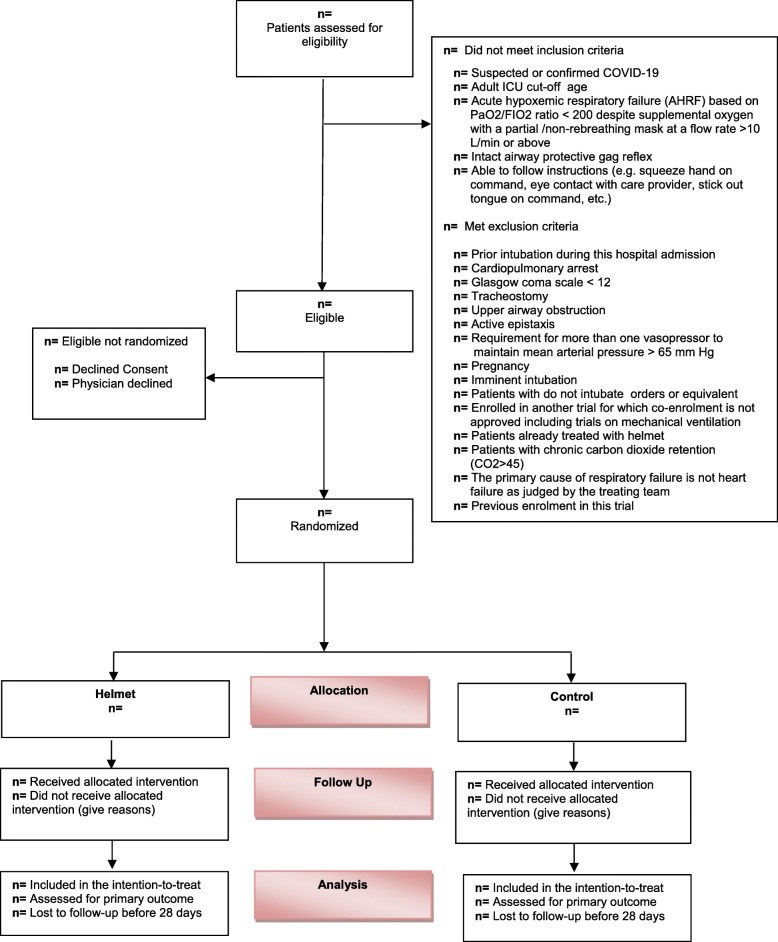
Methods: In this multicenter randomized trial of helmet noninvasive ventilation for COVID-19 patients, 320 adult ICU patients (aged ≥14 years or as per local standards) with suspected or confirmed COVID-19 and acute hypoxemic respiratory failure (ratio of arterial oxygen partial pressure to fraction of inspired oxygen < 200 despite supplemental oxygen with a partial/non-rebreathing mask at a flow rate of 10 L/min or higher) will be randomized to helmet noninvasive ventilation with usual care or usual care alone, which may include mask noninvasive ventilation, high-flow nasal oxygen, or standard oxygen therapy. The primary outcome is death from any cause within 28 days after randomization. The trial has 80% power to detect a 15% absolute risk reduction in 28-day mortality from 40 to 25%. The primary outcome will be compared between the helmet and usual care group in the intention-to-treat using the chi-square test. Results will be reported as relative risk and 95% confidence interval. The first patient was enrolled on February 8, 2021. As of August 1, 2021, 252 patients have been enrolled from 7 centers in Saudi Arabia and Kuwait.
Discussion: We developed a detailed statistical analysis plan to guide the analysis of the Helmet-COVID trial, which is expected to conclude enrollment in November 2021.
Trial registration: ClinicalTrials.gov NCT04477668 . Registered on July 20, 2020.
Keywords: COVID-19; Helmet noninvasive ventilation; Noninvasive ventilation; Statistical analysis plan.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
Arabi YM, Aldekhyl S, Al Qahtani S, Al-Dorzi HM, Abdukahil SA, Al Harbi MK, Al Qasim E, Kharaba A, Albrahim T, Alshahrani MS, Al-Fares AA, Al Bshabshe A, Mady A, Al Duhailib Z, Algethamy H, Jose J, Al Mutairi M, Al Zumai O, Al Haji H, Alaqeily A, Al Aseri Z, Al-Omari A, Al-Dawood A, Tlayjeh H; Saudi Critical Care Trials Group.JAMA. 2022 Sep 20;328(11):1063-1072. doi: 10.1001/jama.2022.15599.PMID: 36125473 Free PMC article. Clinical Trial.
Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, Montomoli J, Falò G, Tonetti T, Cutuli SL, Pintaudi G, Tanzarella ES, Piervincenzi E, Bongiovanni F, Dell'Anna AM, Delle Cese L, Berardi C, Carelli S, Bocci MG, Montini L, Bello G, Natalini D, De Pascale G, Velardo M, Volta CA, Ranieri VM, Conti G, Maggiore SM, Antonelli M; COVID-ICU Gemelli Study Group.JAMA. 2021 May 4;325(17):1731-1743. doi: 10.1001/jama.2021.4682.PMID: 33764378 Free PMC article. Clinical Trial.
Arabi YM, Al-Dorzi HM, Aldekhyl S, Al Qahtani S, Abdukahil SA, Al Qasim E, Al Harbi MK, Kharaba A, Albrahim T, Alshahrani MS, Al-Fares AA, Al Bshabshe A, Mady A, Al Duhailib Z, Algethamy H, Jose J, Al Mutairi M, Al Zumai O, Al Haji H, Alaqeily A, Al Wehaibi W, Al Aseri Z, Al-Omari A, Tlayjeh H, Al-Dawood A; Saudi Critical Care Trials Group.Intensive Care Med. 2023 Mar;49(3):302-312. doi: 10.1007/s00134-023-06981-5. Epub 2023 Feb 23.PMID: 36820878 Free PMC article. Clinical Trial.
Liu Q, Gao Y, Chen R, Cheng Z.Crit Care. 2016 Aug 23;20(1):265. doi: 10.1186/s13054-016-1449-4.PMID: 27549178 Free PMC article. Review.
Advantages and drawbacks of helmet noninvasive support in acute respiratory failure.
Bongiovanni F, Michi T, Natalini D, Grieco DL, Antonelli M.Expert Rev Respir Med. 2023 Jan;17(1):27-39. doi: 10.1080/17476348.2023.2174974. Epub 2023 Feb 9.PMID: 36710082 Review.
Cited by
Arabi YM, Aldekhyl S, Al Qahtani S, Al-Dorzi HM, Abdukahil SA, Al Harbi MK, Al Qasim E, Kharaba A, Albrahim T, Alshahrani MS, Al-Fares AA, Al Bshabshe A, Mady A, Al Duhailib Z, Algethamy H, Jose J, Al Mutairi M, Al Zumai O, Al Haji H, Alaqeily A, Al Aseri Z, Al-Omari A, Al-Dawood A, Tlayjeh H; Saudi Critical Care Trials Group.JAMA. 2022 Sep 20;328(11):1063-1072. doi: 10.1001/jama.2022.15599.PMID: 36125473 Free PMC article. Clinical Trial.
Gasa M, Ruiz-Albert Y, Cordoba-Izquierdo A, Sarasate M, Cuevas E, Suarez-Cuartin G, Méndez L, Alfaro-Álvarez JC, Sabater-Riera J, Pérez-Fernández XL, Molina-Molina M, Santos S.Int J Environ Res Public Health. 2022 Aug 29;19(17):10772. doi: 10.3390/ijerph191710772.PMID: 36078488 Free PMC article.
KMEL References
References
-
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. - DOI - PMC - PubMed
-
- Amirfarzan H, Cereda M, Gaulton T, Leissner K, Cortegiani A, Schumann R, et al. Use of helmet CPAP in COVID-19: a practical review. Pulmonology. 2021;S2531-0437(21):00040–00044.
-
- Cosentini R, Brambilla AM, Aliberti S, Bignamini A, Nava S, Maffei A, Martinotti R, Tarsia P, Monzani V, Pelosi P. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010;138(1):114–120. doi: 10.1378/chest.09-2290. - DOI - PubMed
-
- Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435–2441. doi: 10.1001/jama.2016.6338. - DOI - PMC - PubMed
-
- Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. Jama. 2020;324(1):57–67. doi: 10.1001/jama.2020.9524. - DOI - PMC - PubMed
-
- Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731-43. 10.1001/jama.2021.4682. - PMC - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodol) 1995;57(1):289–300.
-
- Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. doi: 10.1093/biomet/80.3.557. - DOI
-
- EQ-5D-5L Crosswalk Index Value Calculator. Available at: https://euroqol.org/eq-5dinstruments/eq-5d-5l-about/valuation/crosswalk-... 2021.
-
- Muruganandan S, Azzopardi M, Fitzgerald DB, Shrestha R, Kwan BCH, Lam DCL, de Chaneet CC, Rashid Ali MRS, Yap E, Tobin CL, Garske LA, Nguyen PT, Stanley C, Popowicz ND, Kosky C, Thomas R, Read CA, Budgeon CA, Feller-Kopman D, Maskell NA, Murray K, Lee YCG. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6(9):671–680. doi: 10.1016/S2213-2600(18)30288-1. - DOI - PubMed