"Real-life" Efficacy and Safety Aspects of 4-Year Omalizumab Treatment for Asthma
Affiliations
Affiliations
- 1Department of Microbiology, Faculty of Medicine, Kuwait University, Kuwait, Kuwait.
- 2Al-Rashed Allergy Center, Ministry of Health, Kuwait, Kuwait.
- 3National Center of Health Information, Ministry of Health, Kuwait, Kuwait.
- 4Cathedra for Internal Medicine Department, Faculty of Medicine, University of Tuzla, Tuzla, Bosnia-Herzegovina.
Abstract
Objective: To evaluate the long-term efficacy and safety of omalizumab in asthma in a real-life setting.
Subjects and methods: This 4-year observational study included 65 patients treated with omalizumab during clinic visits; treatment response was rated as excellent, good, and partial based on a modified physician's Global Evaluation of Treatment Effectiveness (mGETE) scale of emergency room visits (ERV), hospitalization, use of oral corticosteroids, inhaled corticosteroid (ICS)/long-acting β-agonist (LABA) dose, and short-acting β-agonist rescue. The following tests were done: forced expiratory volume in 1 s (FEV1) and the asthma control test (ACT). Measurements were performed 1 month before therapy and at 16 weeks, 1 year, and 4 years of treatment. Statistical analyses were done using the Wilcoxon signed-rank test, Spearman rank correlation, and McNemar χ2 test.
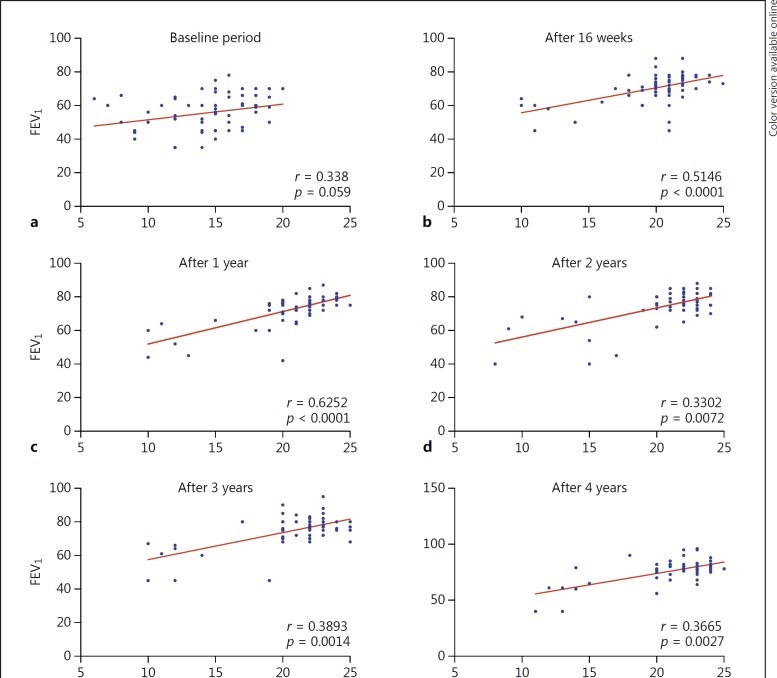
Results: The dropout rate was 15 (18.5%): 8 nonresponders (10.0%); 2 patients died (2.5%), and 5 were lost to follow-up (6.25%). Treatment response was excellent in 35 (53.8%); good in 23 (35.4%), and partial in 7 patients (10.8%). The number of excellent responders increased from 35 (53.8%) at 16 weeks to 48 (73.8%) at the 4-year follow-up. The number of patients who did not require ERV improved from 0 to 59 (90.8%), and the lowest rate of hospitalization was 1 in year 4 (p < 0.001); patients who did not require courses of oral corticosteroids improved from 0 to 54 (83%). ICS/LABA dose significantly reduced from 65 (100%) to 25 (38.5%) after 4 years of treatment (p < 0.001); ACT scores significantly increased from 15 ± 3 at baseline to 23 ± 3 (p < 0.001) and FEV1 level from 55.6 ± 10.6 to 76.63 ± 10.34 at year 4.
Conclusion: In this study, omalizumab therapy resulted in better asthma control, and was effective and well tolerated as an add-on therapy for patients with moderate-to-severe asthma.
Keywords: Asthma; Efficacy; Long-term therapy; Omalizumab; Safety.
Figures
Similar articles
Rubin AS, Souza-Machado A, Andradre-Lima M, Ferreira F, Honda A, Matozo TM; QUALITX Study Investigators.J Asthma. 2012 Apr;49(3):288-93. doi: 10.3109/02770903.2012.660297. Epub 2012 Feb 23.PMID: 22356355 Clinical Trial.
Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, Samara K, Skoula G, Damianaki A, Schiza S, Tzanakis N, Siafakas NM.Pulm Pharmacol Ther. 2012 Feb;25(1):77-82. doi: 10.1016/j.pupt.2011.11.004. Epub 2011 Dec 3.PMID: 22155001
Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G.Respir Med. 2008 Oct;102(10):1371-8. doi: 10.1016/j.rmed.2008.06.002. Epub 2008 Jul 26.PMID: 18657960 Clinical Trial.
Corren J, Kavati A, Ortiz B, Colby JA, Ruiz K, Maiese BA, Cadarette SM, Panettieri RA Jr.Allergy Asthma Proc. 2017 Jul 1;38(4):250-263. doi: 10.2500/aap.2017.38.4067. Epub 2017 Jun 19.PMID: 28631599 Review.
Spotlight on omalizumab in allergic asthma.
Bang LM, Plosker GL.BioDrugs. 2004;18(6):415-8. doi: 10.2165/00063030-200418060-00007.PMID: 15571425 Review.
Cited by
Siracká S, Tinková LD, Hochmuth L, Leščišinová H, Hrubiško M, Dostálová K, Jeseňák M.Postepy Dermatol Alergol. 2023 Feb;40(1):134-141. doi: 10.5114/ada.2022.116532. Epub 2022 Jun 1.PMID: 36909923 Free PMC article.
Hanania NA, Niven R, Chanez P, Antoine D, Pfister P, Garcia Conde L, Jaumont X.World Allergy Organ J. 2022 Sep 25;15(10):100695. doi: 10.1016/j.waojou.2022.100695. eCollection 2022 Oct.PMID: 36254180 Free PMC article. Review.
Menzella F, Fontana M, Contoli M, Ruggiero P, Galeone C, Capobelli S, Simonazzi A, Catellani C, Scelfo C, Castagnetti C, Livrieri F, Facciolongo N.J Asthma Allergy. 2022 Apr 21;15:505-515. doi: 10.2147/JAA.S363398. eCollection 2022.PMID: 35495876 Free PMC article.
Omalizumab: An Optimal Choice for Patients with Severe Allergic Asthma.
Kotoulas SC, Tsiouprou I, Fouka E, Pataka A, Papakosta D, Porpodis K.J Pers Med. 2022 Jan 26;12(2):165. doi: 10.3390/jpm12020165.PMID: 35207654 Free PMC article. Review.
Li Z, Zhang W, Luo F, Li J, Yang W, Zhu B, Wu Q, Wang X, Sun C, Xie Y, Xu B, Wang Z, Qian F, Chen J, Wan Y, Hu W.Front Cell Dev Biol. 2021 Jun 8;9:678377. doi: 10.3389/fcell.2021.678377. eCollection 2021.PMID: 34169075 Free PMC article.