Mutations in Human Accelerated Regions Disrupt Cognition and Social Behavior
Affiliations
Affiliations
- Division of Genetics and Genomics, Manton Center for Orphan Disease Research, and Howard Hughes Medical Institute, Boston Children's Hospital, Boston, MA 02115, USA.
- Department of Molecular Biology, Centro de Biología Molecular 'Severo Ochoa', Universidad Autonoma de Madrid, UAM-CSIC, Nicolas Cabrera 1, 28049 Madrid, Spain; Department of Molecular and Cellular Biology, Centro Nacional de Biotecnología, CNB-CSIC, Darwin 3, Campus de Cantoblanco, 28049 Madrid, Spain.
- Kuwait Center for Autism, 73455 Kuwait City, Kuwait.
- Istanbul Institute of Child and Adolescent Psychiatry, 34365 Istanbul, Turkey.
- Department of Child and Adolescent Psychiatry, Bahcesehir University School of Medicine, 34353 Istanbul, Turkey.
- Division of Genetics and Genomics, Manton Center for Orphan Disease Research, and Howard Hughes Medical Institute, Boston Children's Hospital, Boston, MA 02115, USA; Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, PO Box 17666, Al-Ain, United Arab Emirates.
- Department of Pediatrics, King Faisal Specialist Hospital and Research Center, Jeddah 21499, Kingdom of Saudi Arabia.
- Department of Neurology (Pediatric Neurology), Massachusetts General Hospital, Boston, MA 02114, USA.
- Department of Molecular and Cellular Biology, Centro Nacional de Biotecnología, CNB-CSIC, Darwin 3, Campus de Cantoblanco, 28049 Madrid, Spain.
- Division of Genetics and Genomics, Manton Center for Orphan Disease Research, and Howard Hughes Medical Institute, Boston Children's Hospital, Boston, MA 02115, USA; Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA; Departments of Pediatrics and Neurology, Harvard Medical School, Boston, MA 02115, USA. Electronic address: christopher.walsh@childrens.harvard.edu.
Abstract
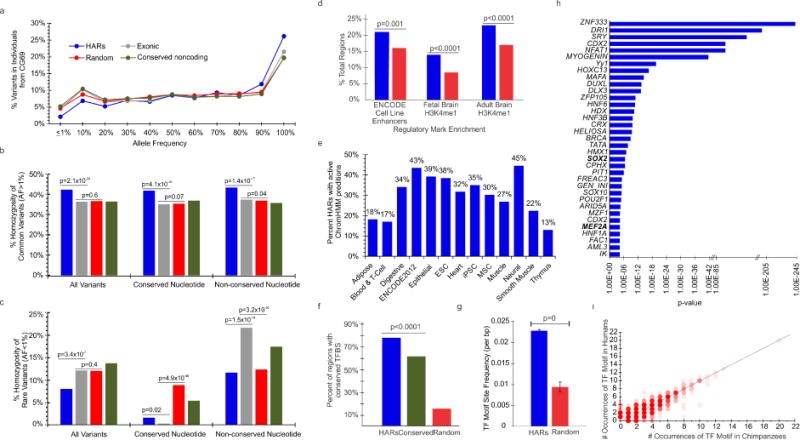
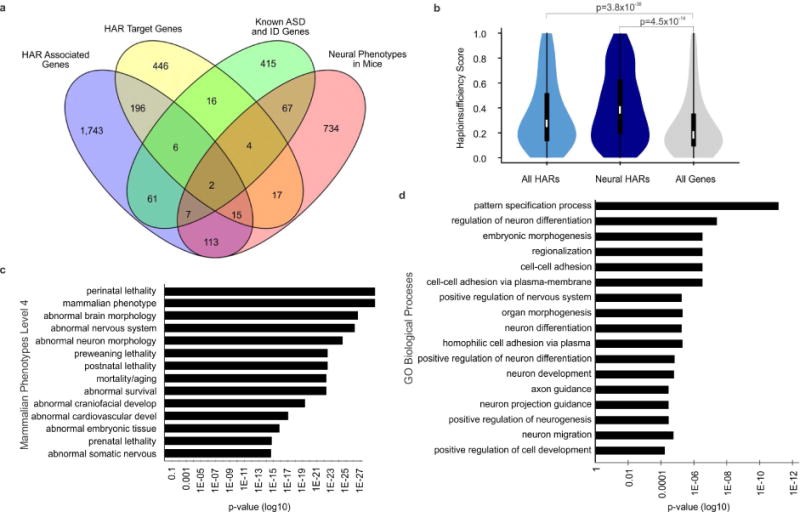
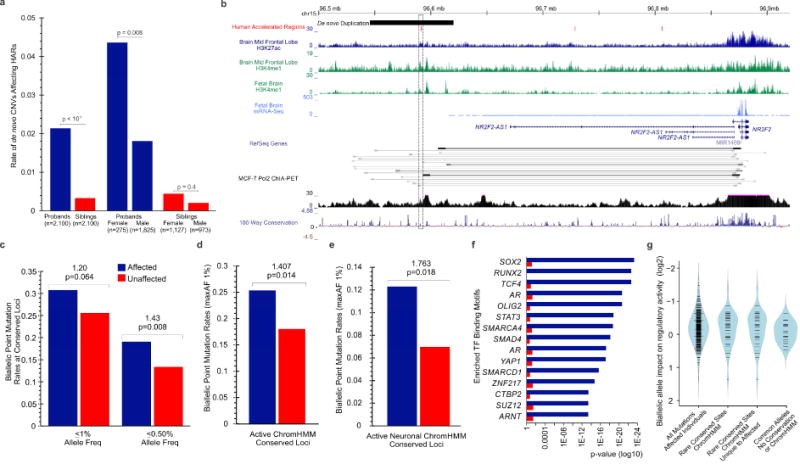
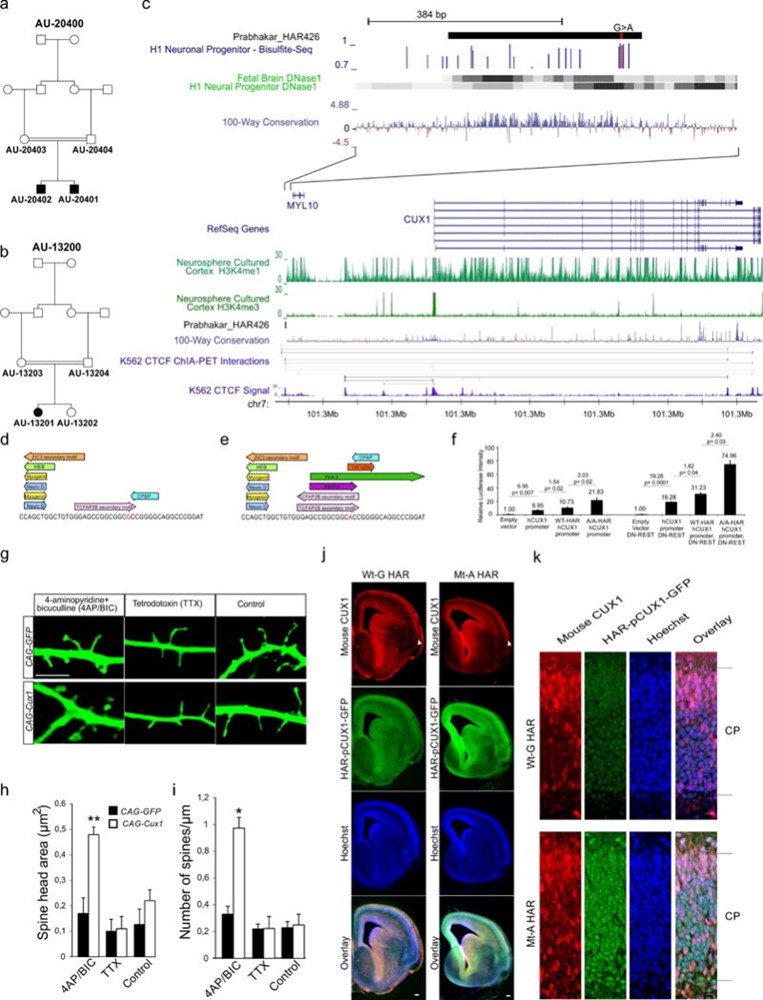
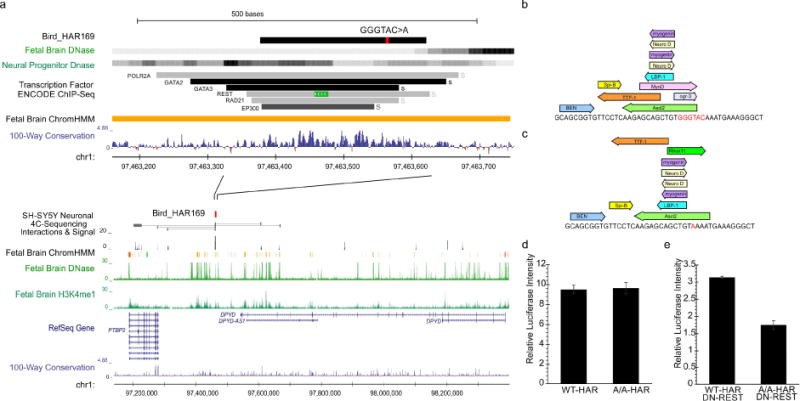
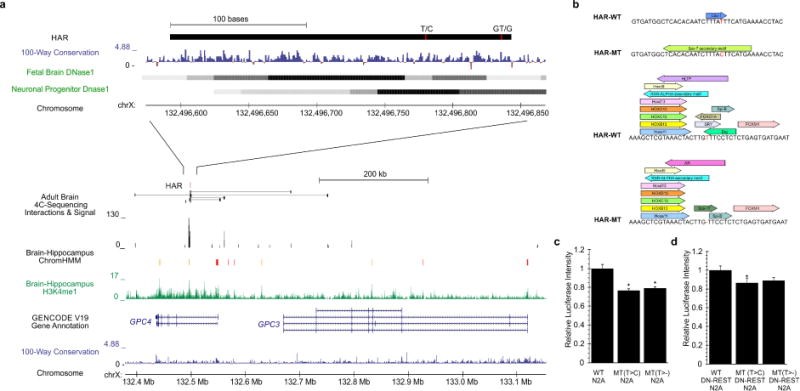
Comparative analyses have identified genomic regions potentially involved in human evolution but do not directly assess function. Human accelerated regions (HARs) represent conserved genomic loci with elevated divergence in humans. If some HARs regulate human-specific social and behavioral traits, then mutations would likely impact cognitive and social disorders. Strikingly, rare biallelic point mutations-identified by whole-genome and targeted "HAR-ome" sequencing-showed a significant excess in individuals with ASD whose parents share common ancestry compared to familial controls, suggesting a contribution in 5% of consanguineous ASD cases. Using chromatin interaction sequencing, massively parallel reporter assays (MPRA), and transgenic mice, we identified disease-linked, biallelic HAR mutations in active enhancers for CUX1, PTBP2, GPC4, CDKL5, and other genes implicated in neural function, ASD, or both. Our data provide genetic evidence that specific HARs are essential for normal development, consistent with suggestions that their evolutionary changes may have altered social and/or cognitive behavior. PAPERCLIP.
Keywords: ASD; Autism; Brain Evolution; HARs; Human Accelerated regions; noncoding.
Figures
Similar articles
Genetic variations in evolutionary accelerated regions disrupt cognition in schizophrenia.
Bhattacharyya U, Bhatia T, Deshpande SN, Thelma BK.Psychiatry Res. 2022 Aug;314:114586. doi: 10.1016/j.psychres.2022.114586. Epub 2022 May 13.PMID: 35623238
Cantor RM, Navarro L, Won H, Walker RL, Lowe JK, Geschwind DH.Mol Psychiatry. 2018 Apr;23(4):993-1000. doi: 10.1038/mp.2017.114. Epub 2017 May 23.PMID: 28533516 Free PMC article.
Chen R, Davis LK, Guter S, Wei Q, Jacob S, Potter MH, Cox NJ, Cook EH, Sutcliffe JS, Li B.Mol Autism. 2017 Mar 21;8:14. doi: 10.1186/s13229-017-0130-3. eCollection 2017.PMID: 28344757 Free PMC article.
Guardiola-Ripoll M, Fatjó-Vilas M.Int J Mol Sci. 2023 Feb 10;24(4):3597. doi: 10.3390/ijms24043597.PMID: 36835010 Free PMC article. Review.
Le Gall E, Iakimova G.Encephale. 2018 Dec;44(6):523-537. doi: 10.1016/j.encep.2018.03.004. Epub 2018 Aug 16.PMID: 30122298 Review. French.
Cited by
Evolutionarily recent retrotransposons contribute to schizophrenia.
Modenini G, Abondio P, Guffanti G, Boattini A, Macciardi F.Transl Psychiatry. 2023 May 27;13(1):181. doi: 10.1038/s41398-023-02472-9.PMID: 37244930 Free PMC article.
Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor.
Eichwald T, da Silva LB, Staats Pires AC, Niero L, Schnorrenberger E, Filho CC, Espíndola G, Huang WL, Guillemin GJ, Abdenur JE, Latini A.Antioxidants (Basel). 2023 May 3;12(5):1037. doi: 10.3390/antiox12051037.PMID: 37237903 Free PMC article. Review.
The brain on time: links between development and neurodegeneration.
Shabani K, Hassan BA.Development. 2023 May 15;150(10):dev200397. doi: 10.1242/dev.200397. Epub 2023 May 15.PMID: 37184296
Leveraging primate-specific genomic information for genetic studies of complex diseases.
Wei WH, Guo H.Front Bioinform. 2023 Mar 28;3:1161167. doi: 10.3389/fbinf.2023.1161167. eCollection 2023.PMID: 37056664 Free PMC article.
Khatoon H, Raza RZ, Saleem S, Batool F, Arshad S, Abrar M, Ali S, Hussain I, Shubin NH, Abbasi AA.BMC Mol Cell Biol. 2023 Mar 29;24(1):13. doi: 10.1186/s12860-023-00474-5.PMID: 36991330 Free PMC article.