Confirmed disability progression as a marker of permanent disability in multiple sclerosis
Sifat Sharmin 1, Francesca Bovis 2, Charles Malpas 1 3, Dana Horakova 4, Eva Kubala Havrdova 4, Guillermo Izquierdo 5, Sara Eichau 5, Maria Trojano 6, Alexandre Prat 7 8, Marc Girard 7 8, Pierre Duquette 7 8, Marco Onofrj 9, Alessandra Lugaresi 10 11, Francois Grand'Maison 12, Pierre Grammond 13, Patrizia Sola 14, Diana Ferraro 14, Murat Terzi 15, Oliver Gerlach 16, Raed Alroughani 17, Cavit Boz 18, Vahid Shaygannejad 19, Vincent van Pesch 20 21, Elisabetta Cartechini 22, Ludwig Kappos 23, Jeannette Lechner-Scott 24 25, Roberto Bergamaschi 26, Recai Turkoglu 27, Claudio Solaro 28, Gerardo Iuliano 29, Franco Granella 30 31, Bart Van Wijmeersch 32, Daniele Spitaleri 33, Mark Slee 34, Pamela McCombe 35 36, Julie Prevost 37, Radek Ampapa 38, Serkan Ozakbas 39, Jose Luis Sanchez-Menoyo 40, Aysun Soysal 41, Steve Vucic 42, Thor Petersen 43, Koen de Gans 44, Ernest Butler 45, Suzanne Hodgkinson 46, Youssef Sidhom 47, Riadh Gouider 47, Edgardo Cristiano 48, Tamara Castillo-Triviño 49, Maria Laura Saladino 50, Michael Barnett 51, Fraser Moore 52, Csilla Rozsa 53, Bassem Yamout 54, Olga Skibina 55 56, Anneke van der Walt 55 56, Katherine Buzzard 55 56, Orla Gray 57, Stella Hughes 58, Angel Perez Sempere 59, Bhim Singhal 60, Yara Fragoso 61, Cameron Shaw 62, Allan Kermode 63 64 65, Bruce Taylor 66, Magdolna Simo 67, Neil Shuey 68, Talal Al-Harbi 69, Richard Macdonell 70, Jose Andres Dominguez 71, Tunde Csepany 72, Carmen Adella Sirbu 73, Maria Pia Sormani 2, Helmut Butzkueven 55 56, Tomas Kalincik 1 3
Affiliations
Affiliations
- 1CORe, Department of Medicine, University of Melbourne, Melbourne, Australia.
- 2Department of Health Sciences (DISSAL), University of Genoa, Genoa, Italy.
- 3Melbourne MS Centre, Department of Neurology, Royal Melbourne Hospital, Melbourne, Australia.
- 4Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine, Charles University in Prague and General University Hospital, Prague, Czech Republic.
- 5Hospital Universitario Virgen Macarena, Sevilla, Spain.
- 6Department of Basic Medical Sciences, Neuroscience and Sense Organs, University of Bari, Bari, Italy.
- 7Hopital Notre Dame, Montreal, QC, Canada.
- 8CHUM and Universite de Montreal, Montreal, QC, Canada.
- 9Department of Neuroscience, Imaging, and Clinical Sciences, University G. d'Annunzio, Chieti, Italy.
- 10IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italia.
- 11Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Bologna, Italia.
- 12Neuro Rive-Sud, Greenfield Park, QC, Canada.
- 13CISSS Chaudière-Appalache, Levis, QC, Canada.
- 14Department of Neuroscience, Azienda Ospedaliera Universitaria, Modena, Italy.
- 15Medical Faculty, 19 Mayis University, Samsun, Turkey.
- 16Department of Neurology, Zuyderland Medical Center, Sittard-Geleen, The Netherlands.
- 17Division of Neurology, Department of Medicine, Amiri Hospital, Sharq, Kuwait.
- 18KTU Medical Faculty Farabi Hospital, Trabzon, Turkey.
- 19Isfahan University of Medical Sciences, Isfahan, Iran.
- 20Cliniques Universitaires Saint-Luc, Brussels, Belgium.
- 21Université Catholique de Louvain, Louvain-la-Neuve, Belgium.
- 22UOC Neurologia, Azienda Sanitaria Unica Regionale Marche - AV3, Macerata, Italy.
- 23Neurologic Clinic and Policlinic, Departments of Medicine and Clinical Research, University Hospital and University of Basel, Basel, Switzerland.
- 24School of Medicine and Public Health, University Newcastle, Newcastle, NSW, Australia.
- 25Department of Neurology, John Hunter Hospital, Hunter New England Health, Newcastle, NSW, Australia.
- 26IRCCS Mondino Foundation, Pavia, Italy.
- 27Haydarpasa Numune Training and Research Hospital, Istanbul, Turkey.
- 28Department of Rehabilitaiton, ML Novarese Hospital, Moncrivello, Italy.
- 29Ospedali Riuniti di Salerno, Salerno, Italy.
- 30Department of Medicine and Surgery, University of Parma, Parma, Italy.
- 31Department of General Medicine, Parma University Hospital, Parma, Italy.
- 32Rehabilitation and MS-Centre Overpelt and Hasselt University, Hasselt, Belgium.
- 33Azienda Ospedaliera di Rilievo Nazionale San Giuseppe Moscati Avellino, Avellino, Italy.
- 34Flinders University, Adelaide, SA, Australia.
- 35University of Queensland, Brisbane, Qld, Australia.
- 36Royal Brisbane and Women's Hospital, Brisbane, Qld, Australia.
- 37CSSS Saint-Jerome, Saint-Jérôme, QC, Canada.
- 38Nemocnice Jihlava, Jihlava, Czech Republic.
- 39Dokuz Eylul University, Konak/Izmir, Turkey.
- 40Hospital de Galdakao-Usansolo, Galdakao, Spain.
- 41Bakirkoy Education and Research Hospital for Psychiatric and Neurological Diseases, Istanbul, Turkey.
- 42Westmead Hospital, Sydney, NSW, Australia.
- 43University hospital Aarhus, Aarhus, Denmark.
- 44Groene Hart Ziekenhuis, Gouda, The Netherlands.
- 45Monash Medical Centre, Melbourne, Vic., Australia.
- 46Liverpool Hospital, Sydney, NSW, Australia.
- 47Department of Neurology, Razi Hospital, Manouba, Tunisia.
- 48Hospital Italiano, Buenos Aires, Argentina.
- 49Instituto de Investigación Sanitaria Biodonostia, Department of Neurology, Hospital Universitario Donostia, San Sebastián, Spain.
- 50INEBA - Institute of Neuroscience Buenos Aires, Buenos Aires, Argentina.
- 51Brain and Mind Centre, Sydney, NSW, Australia.
- 52Jewish General Hospital, Montreal, QC, Canada.
- 53Jahn Ferenc Teaching Hospital, Budapest, Hungary.
- 54Nehme and Therese Tohme Multiple Sclerosis Center, American University of Beirut Medical Center, Beirut, Lebanon.
- 55Central Clinical School, Monash University, Melbourne, Vic., Australia.
- 56Department of Neurology, The Alfred Hospital, Melbourne, Vic., Australia.
- 57South East Trust, Belfast, UK.
- 58Craigavon Area Hospital, Craigavon, UK.
- 59Hospital General Universitario de Alicante, Alicante, Spain.
- 60Bombay Hospital Institute of Medical Sciences, Mumbai, India.
- 61Universidade Metropolitana de Santos, Santos, Brazil.
- 62Geelong Hospital, Geelong, Vic., Australia.
- 63Perron Institute, University of Western Australia, Nedlands, WA, Australia.
- 64Institute of Immunology and Infectious Diseases, Murdoch University, Perth, WA, Australia.
- 65Sir Charles Gairdner Hospital, Nedlands, WA, Australia.
- 66Royal Hobart Hospital, Hobart, TAS, Australia.
- 67Semmelweis University Budapest, Budapest, Hungary.
- 68St Vincents Hospital, Fitzroy, Melbourne, Vic., Australia.
- 69Neurology Department, King Fahad Specialist Hospital-Dammam, Dammam, Saudi Arabia.
- 70Austin Health, Melbourne, Vic., Australia.
- 71Hospital Universitario de la Ribera, Alzira, Spain.
- 72Department of Neurology, Faculty of Medicine, University of Debrecen, Debrecen, Hungary.
- 73Central Military Emergency University Hospital, Titu Maiorescu University, Bucharest, Romania.
Abstract
Background and purpose: The prevention of disability over the long term is the main treatment goal in multiple sclerosis (MS); however, randomized clinical trials evaluate only short-term treatment effects on disability. This study aimed to define criteria for 6-month confirmed disability progression events of MS with a high probability of resulting in sustained long-term disability worsening.
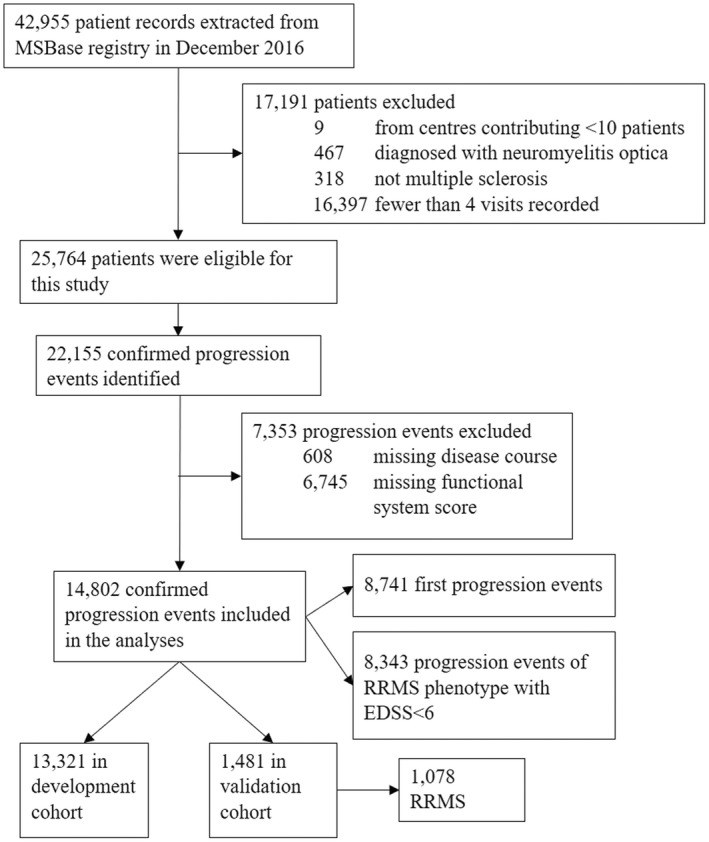
Methods: In total, 14,802 6-month confirmed disability progression events were identified in 8741 patients from the global MSBase registry. For each 6-month confirmed progression event (13,321 in the development and 1481 in the validation cohort), a sustained progression score was calculated based on the demographic and clinical characteristics at the time of progression that were predictive of long-term disability worsening. The score was externally validated in the Cladribine Tablets Treating Multiple Sclerosis Orally (CLARITY) trial.
Results: The score was based on age, sex, MS phenotype, relapse activity, disability score and its change from baseline, number of affected functional system domains and worsening in six of the domains. In the internal validation cohort, a 61% lower chance of improvement was estimated with each unit increase in the score (hazard ratio 0.39, 95% confidence interval 0.29-0.52; discriminatory index 0.89). The proportions of progression events sustained at 5 years stratified by the score were 1: 72%; 2: 88%; 3: 94%; 4: 100%. The results of the CLARITY trial were confirmed for reduction of disability progression that was >88% likely to be sustained (events with score ˃1.5).
Conclusions: Clinicodemographic characteristics of 6-month confirmed disability progression events identify those at high risk of sustained long-term disability. This knowledge will allow future trials to better assess the effect of therapy on long-term disability accrual.
Keywords: CLARITY; clinical trial; functional system impairment; risk scoring; sustained disability progression.
Conflict of interest statement
The authors report the following relationships: speaker honoraria, advisory board or steering committee fees, research support and/or conference travel support from Almirall (G.Iz., M.Tr., R.B., C.So., J.L.SM), Bayer (P.S., V.V.P., L.K., J.LS., R.B., G.Iu., MSl, RAl, RAm, J.L.SM, T.P., S.H., C.R., B.T., M.Si., N.S., P.M., T.C., S.E., M.Tr., M.Te., E.Cr.), BioCSL (T.K., K.B.), Biogen (A.L., D.H., P.G., P.S., D.F., H.B., J.LS., F.Gm., F.Gr., S.H., M.B., O.Gr., B.S., C.So., C.Sh., B.T., N.S., P.M., M.P.S., O.Ge., O.S., S.E., K.B., M.Tr., G.Iz., G.Iu., MSl, M.Si., RAl, R.Am., E.Cr.), Celgene (E.K.H.), Genzyme‐Sanofi (T.K., A.L., M.P.S., O.Gr., O.Ge., O.S., S.E., K.B., M.Tr., M.Te., C.So., G.Iz., G.Iu., F.Gm., F.Gr., MSl, RAl, E.Cr.), GSK (RAl), Innate Immunotherapeutics (A.K.), Merck/EMD (D.H., E.K.H., G.Iu., M.Tr., A.L., P.G., P.S., D.F., T.K., M.Te., R.Am., H.B., C.B., V.V.P., L.K., J.LS, R.B., C.So., G.Iz., F.Gr., B.V.W., D.S., MSl, RAl, J.L.SM., T.P., S.Ho., E.Cr., M.B., C.R., O.Gr., B.S., Y.F., A.K., M.Si., T.C., M.G., P.D., F.M., M.P.S., O.Ge., O.S., S.E., K.B.), Mitsubishi (F.Gm.), Mylan (A.L.), Novartis (D.H., E.K.H., G.Iz., M.Tr., M.G., P.D., A.L., F.Gm., P.G., P.S., D.F., T.K., M.Te., R.Am., H.B., CB, V.V.P., L.K., J.LS., R.B., C.So., G.Iu., F.Gr., B.V.W., D.S., MSl, J.P., RAl, J.L.SM, T.P., S.H., E.Cr., M.B., F.M., C.R., O.Gr., Y.F., C.Sh., A.K., BT, MSi, NV, NS, PM, TC, MPS, OS, FB, SE, K.B.), ONO Pharmaceuticals (FGm), Roche (DH, EKH, GIz, AL, TK, MTe, RAl, CB, VVP, LK, FGr, BVW, T.P., ECr, Y.F., B.T., T.C., M.P.S., S.Ho., S.E., K.B.), Teva (D.H., E.K.H., G.Iz., G.Iu., M.Tr., M.G., P.D., A.L., F.Gm., P.G., P.S., D.F., T.K., M.Te., R.T., C.B., V.V.P., L.K., J.LS., R.B., C.So., B.V.W., D.S., J.P., R.Am., J.L.SM., C.R., Y.F., A.K., M.Si., T.C., C.A.S., M.P.S., O.Ge., O.S., S.E., K.B.), WebMD (T.K.), UCB (L.K.), GeNeuro (M.P.S.), Medday (M.P.S.), Fondazione Italiana Sclerosi Multipla (A.L.), Grifols (K.B.), Actelion (R.Am.).
Figures
Similar articles
Giovannoni G, Comi G, Rammohan K, Rieckmann P, Dangond F, Keller B, Jack D, Vermersch P.Adv Ther. 2021 Sep;38(9):4975-4985. doi: 10.1007/s12325-021-01865-w. Epub 2021 Aug 9.PMID: 34370275 Free PMC article. Clinical Trial.
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett A, Viglietta V, Greenberg S; CLARITY study group.Lancet Neurol. 2011 Apr;10(4):329-37. doi: 10.1016/S1474-4422(11)70023-0.PMID: 21397565
Filippini G, Del Giovane C, Clerico M, Beiki O, Mattoscio M, Piazza F, Fredrikson S, Tramacere I, Scalfari A, Salanti G.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2.PMID: 28440858 Free PMC article. Review.
Defining reliable disability outcomes in multiple sclerosis.
Kalincik T, Cutter G, Spelman T, Jokubaitis V, Havrdova E, Horakova D, Trojano M, Izquierdo G, Girard M, Duquette P, Prat A, Lugaresi A, Grand'Maison F, Grammond P, Hupperts R, Oreja-Guevara C, Boz C, Pucci E, Bergamaschi R, Lechner-Scott J, Alroughani R, Van Pesch V, Iuliano G, Fernandez-Bolaños R, Ramo C, Terzi M, Slee M, Spitaleri D, Verheul F, Cristiano E, Sánchez-Menoyo JL, Fiol M, Gray O, Cabrera-Gomez JA, Barnett M, Butzkueven H.Brain. 2015 Nov;138(Pt 11):3287-98. doi: 10.1093/brain/awv258. Epub 2015 Sep 10.PMID: 26359291
Mitoxantrone: a review of its use in multiple sclerosis.
Scott LJ, Figgitt DP.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010.PMID: 15089110 Review.
KMEL References
References
-
- CAMMS223 Trial Investigators . Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786‐1801. - PubMed
-
- Polman CH, O'connor PW, Havrdova E, et al. A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899‐910. - PubMed
-
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta‐1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911‐923. - PubMed
-
- Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(11):3287‐3298. - PubMed
-
- Scott T, Wang P, You X, Mann M, Sperling B. Relationship between sustained disability progression and functional system scores in relapsing–remitting multiple sclerosis: analysis of placebo data from four randomized clinical trials. Neuroepidemiology. 2015;44(1):16‐23. - PubMed
-
- Scott T, You X, Foulds P. Functional system scores provide a window into disease activity occurring during a multiple sclerosis treatment trial. Neurol Res. 2011;33(5):549‐552. - PubMed
-
- Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler J. 2006;12(6):769‐774. - PubMed
-
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005;58(6):840‐846. - PubMed
-
- Kalincik T, Kuhle J, Pucci E, et al. Data quality evaluation for observational multiple sclerosis registries. Mult Scler J. 2017;23(5):647‐655. - PubMed
-
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361‐387. - PubMed
-
- Giovannoni G, Comi G, Cook S, et al. A placebo‐controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416‐426. - PubMed
-
- R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018.
-
- Belachew S, Phan‐Ba R, Bartholomé E, et al. Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing–remitting multiple sclerosis. Eur J Neurol. 2011;18(2):240‐245. - PubMed
-
- Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing–remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271‐281. - PubMed
-
- Giovannoni G, Cutter G, Sormani MP, et al. Is multiple sclerosis a length‐dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord. 2017;12:70‐78. - PubMed
-
- Hirst CL, Ingram G, Swingler R, Compston A, Pickersgill TP, Robertson NP. Change in disability in patients with multiple sclerosis: a 20 year prospective population based analysis. J Neurol Neurosurg Psychiatry. 2008;79:1137‐1143. - PubMed
-
- D'Souza M, Yaldizli Ö, John R, et al. Neurostatus e‐scoring improves consistency of Expanded Disability Status Scale assessments: a proof of concept study. Mult Scler J. 2017;23(4):597‐603. - PubMed
-
- Amato MP, Fratiglioni L, Groppi C, Siracusa G, Amaducci L. Interrater reliability in assessing functional systems and disability on the Kurtzke scale in multiple sclerosis. Arch Neurol. 1988;45(7):746‐748. - PubMed
![FIGURE 2 (a) Histogram of the sustained progression scores in the internal validation cohort. n represents the number of patients with each integer progression score. (b) The risk of 6‐month confirmed progression events being sustained over time stratified by the sustained progression score in the internal validation cohort. Example: A progression event confirmed over 6 months was recorded in a 40‐year‐old male diagnosed with RRMS, who presented with a two‐step increase in EDSS from step 5.5 to 7.5, in the absence of a relapse during the preceding month, with five neurological domains affected and worsening in pyramidal (1 unit), cerebellar (2 units) and sensory (2 units) functional system scores. The sustained progression score in this patient was estimated as 2.001 [Colour figure can be viewed at wileyonlinelibrary.com]
show full caption](/Images/Figures/9_449.jpeg)