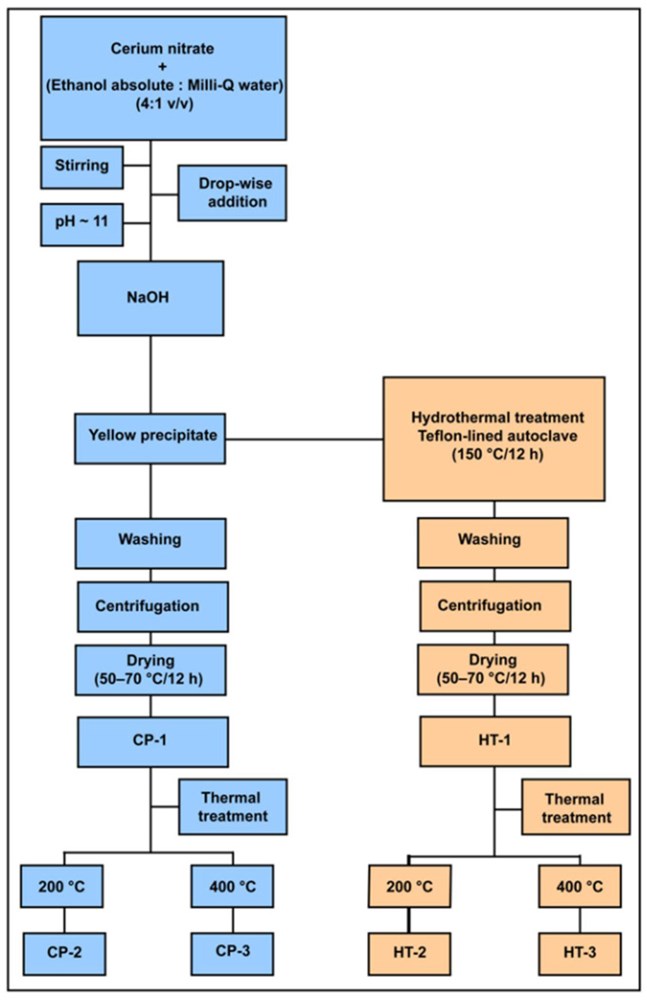
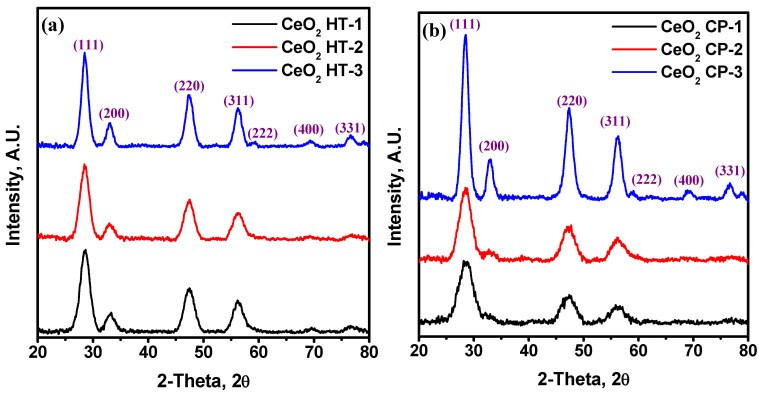
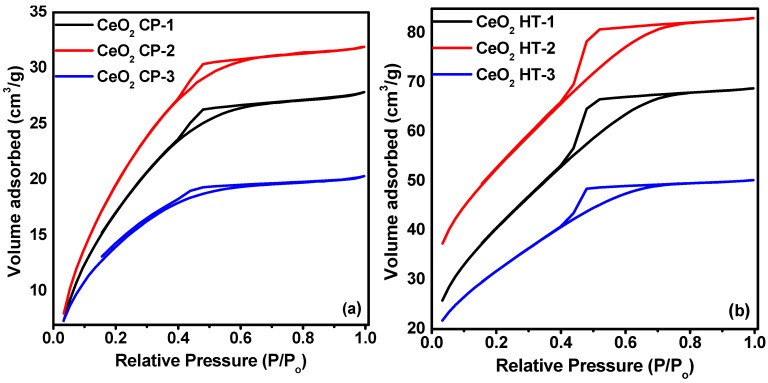
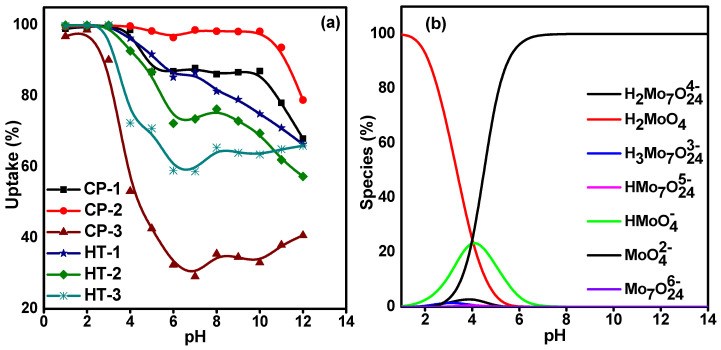
Sorption Profile of Low Specific Activity 99Mo on Nanoceria-Based Sorbents for the Development of 99mTc Generators: Kinetics, Equilibrium, and Thermodynamic Studies
Affiliations
Affiliations
- Department of Chemistry, Biochemistry and Pharmaceutical Sciences, Faculty of Science, University of Bern, Freiestrasse 3, CH-3012 Bern, Switzerland.
- Radioactive Isotopes and Generators Department, Hot Laboratories Center, Egyptian Atomic Energy Authority, Cairo 13759, Egypt.
- Chemistry Department, Faculty of Science, Kuwait University, Safat 13060, Kuwait.
Abstract
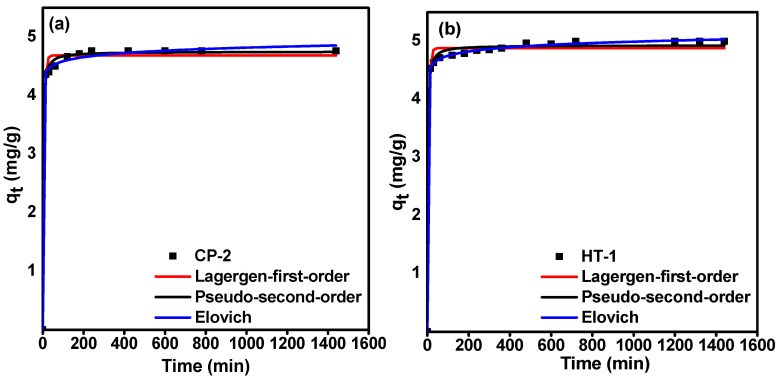
99Mo/99mTc generators play a significant role in supplying 99mTc for diagnostic interventions in nuclear medicine. However, the applicability of using low specific activity (LSA) 99Mo asks for sorbents with high sorption capacity. Herein, this study aims to evaluate the sorption behavior of LSA 99Mo towards several CeO2 nano-sorbents developed in our laboratory. These nanomaterials were prepared by wet chemical precipitation (CP) and hydrothermal (HT) approaches. Then, they were characterized using XRD, BET, FE-SEM, and zeta potential measurements. Additionally, we evaluated the sorption profile of carrier-added (CA) 99Mo onto each material under different experimental parameters. These parameters include pH, initial concentration of molybdate solution, contact time, and temperature. Furthermore, the maximum sorption capacities were evaluated. The results reveal that out of the synthesized CeO2 nanoparticles (NPs) materials, the sorption capacity of HT-1 and CP-2 reach 192 ± 10 and 184 ± 12 mg Mo·g-1, respectively. For both materials, the sorption kinetics and isotherm data agree with the Elovich and Freundlich models, respectively. Moreover, the diffusion study demonstrates that the sorption processes can be described by pore diffusion (for HT-synthesis route 1) and film diffusion (for CP-synthesis route 2). Furthermore, the thermodynamic parameters indicate that the Mo sorption onto both materials is a spontaneous and endothermic process. Consequently, it appears that HT-1 and CP-2 have favorable sorption profiles and high sorption capacities for CA-99Mo. Therefore, they are potential candidates for producing a 99Mo/99mTc radionuclide generator by using LSA 99Mo.
Keywords: CeO2 NPs; LSA 99Mo; hydrothermal modification; sorption kinetics; thermodynamic parameters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
Nawar MF, El-Daoushy AF, Ashry A, Türler A.Molecules. 2022 Sep 2;27(17):5667. doi: 10.3390/molecules27175667.PMID: 36080438 Free PMC article.
Chakravarty R, Ram R, Dash A, Pillai MR.Nucl Med Biol. 2012 Oct;39(7):916-22. doi: 10.1016/j.nucmedbio.2012.03.010. Epub 2012 May 25.PMID: 22632898
Wang J, Gao R, Huang Q, Yin X, Lin M, Cao S, Chen D, Fan F, Wu X, Qin Z, Guo Z, Bai J, Chu J, Tian W, Tan C, Li B, Cheng N, Jia Z.Appl Radiat Isot. 2021 Dec;178:109986. doi: 10.1016/j.apradiso.2021.109986. Epub 2021 Oct 16.PMID: 34673479
Nawar MF, Türler A.Front Chem. 2022 Jul 22;10:926258. doi: 10.3389/fchem.2022.926258. eCollection 2022.PMID: 35936080 Free PMC article. Review.
99Mo/(99m)Tc separation: an assessment of technology options.
Dash A, Knapp FF Jr, Pillai MR.Nucl Med Biol. 2013 Feb;40(2):167-76. doi: 10.1016/j.nucmedbio.2012.10.005. Epub 2012 Nov 9.PMID: 23142410 Review.
Cited by
Nawar MF, El-Daoushy AF, Ashry A, Türler A.Molecules. 2022 Sep 2;27(17):5667. doi: 10.3390/molecules27175667.PMID: 36080438 Free PMC article.
KMEL References
References
-
- Martini P., Boschi A., Cicoria G., Zagni F., Corazza A., Uccelli L., Pasquali M., Pupillo G., Marengo M., Loriggiola M., et al. In-house cyclotron production of high-purity Tc-99m and Tc-99m radiopharmaceuticals. Appl. Radiat. Isot. 2018;139:325–331. doi: 10.1016/j.apradiso.2018.05.033. - DOI - PubMed
-
- Chang S.H. Types of bulk liquid membrane and its membrane resistance in heavy metal removal and recovery from wastewater. Desalin. Water Treat. 2016;57:19785–19793. doi: 10.1080/19443994.2015.1102772. - DOI
-
- I.A.E.A. Radiotracer Generators for Industrial Applications. International Atomic Energy Agency; Vienna, Austria: 2013.
-
- Molinski V.J. A review of 99mTc generator technology. Int. J. Appl. Rad. Isot. 1982;33:811–819. doi: 10.1016/0020-708X(82)90122-3. - DOI
-
- I.A.E.A. Cyclotron Based Production of Technetium-99m. International Atomic Energy Agency; Vienna, Austria: 2017.
-
- Nawar M.F., Türler A. Development of New Generation of 99Mo/99mTc Radioisotope Generators to Meet the Continuing Clinical Demands; Proceedings of the 2nd International Conference on Radioanalytical and Nuclear Chemistry (RANC 2019); Budapest, Hungary. 5–10 May 2019.
-
- Qaim S.M. The present and future of medical radionuclide production. Radiochim. Acta. 2012;100:635–651. doi: 10.1524/ract.2012.1966. - DOI
-
- I.A.E.A. Non-HEU Production Technologies for Molybdenum-99 and Technetium-99m. International Atomic Energy Agency; Vienna, Austria: 2013.
-
- I.A.E.A. Feasibility of Producing Molybdenum-99 on a Small Scale Using Fission of Low Enriched Uranium or Neutron Activation of Natural Molybdenum. International Atomic Energy Agency; Vienna, Austria: 2015.
-
- Maoliang L. Production of gel-type Tc-99m generator for nuclear medicine; Proceedings of the 12th KAIF/KNS Annual Conference; Seoul, Korea. 3–4 April 1997.
-
- Mostafa M., Saber H.M., El-Sadek A.A., Nassar M.Y. Preparation and performance of 99Mo/99mTc chromatographic column generator based on zirconium molybdosilicate. Radiochim. Acta. 2016;104:257–265. doi: 10.1515/ract-2015-2488. - DOI
-
- Sekimoto S., Tatenuma K., Suzuki Y., Tsuguchi A., Tanaka A., Tadokoro T., Kani Y., Morikawa Y., Yamamoto A., Ohtsuki T. Separation and purification of 99mTc from 99Mo produced by electron linear accelerator. J. Radioanal. Nucl. Chem. 2017;311:1361–1366. doi: 10.1007/s10967-016-4959-2. - DOI
-
- Jadhav A. Wet Chemical Methods for Nanop article Synthesis. In: Singh V.N., editor. Chemical Methods for Processing Nanomaterials. 1st ed. CRC Press; Boca Raton, FL, USA: 2021. pp. 49–58.
-
- Baig N., Kammakakam I., Falath W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021;2:1821–1871. doi: 10.1039/D0MA00807A. - DOI
-
- Priya S.D., Nesaraj A.S., Selvakumar A.I. Facile wet-chemical synthesis and evaluation of physico-chemical characteristics of novel nanocrystalline NdCoO3-based perovskite oxide as cathode for LT-SOFC applications. Bull. Mater. Sci. 2021;44:115. doi: 10.1007/s12034-021-02410-9. - DOI
-
- Gan Y.X., Jayatissa A.H., Yu Z., Chen X., Li M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020;2020:8917013. doi: 10.1155/2020/8917013. - DOI
-
- Kigozi M., Ezealigo B.N., Onwualu A.P., Dzade N.Y. Hydrothermal Synthesis of Metal Oxide Composite Cathode Materials for High Energy Application. In: Ezema F.I., Lokhande C.D., Rajan J., editors. Chemically Deposited Nanocrystalline Metal Oxide Thin Films: Synthesis, Characterizations, and Applications. 1st ed. Springer International Publishing; Cham, Switzerland: 2021. pp. 489–508. - DOI
-
- Sakr T.M., Nawar M.F., Fasih T., El-Bayoumy S., Abd El-Rehim H.A. Nano-technology contributions towards the development of high performance radioisotope generators: The future promise to meet the continuing clinical demand. Appl. Radiat. Isot. 2017;129:67–75. doi: 10.1016/j.apradiso.2017.08.012. - DOI - PubMed
-
- Madkour M., Allam O.G., Abdel Nazeer A., Amin M.O., Al-Hetlani E. CeO2-based nanoheterostructures with p–n and n–n heterojunction arrangements for enhancing the solar-driven photodegradation of rhodamine 6G dye. J. Mater. Sci. Mater. Electron. 2019;30:10857–10866. doi: 10.1007/s10854-019-01429-3. - DOI
-
- Ivanets A., Kitikova N., Shashkova I., Radkevich A., Stepanchuk T., Maslova M., Mudruk N. One-Stage Adsorption Treatment of Liquid Radioactive Wastes with Complex Radionuclide Composition. Water Air Soil Pollut. 2020;231:144. doi: 10.1007/s11270-020-04529-7. - DOI
-
- Metwally S.S., Attallah M.F. Impact of surface modification of chabazite on the sorption of iodine and molybdenum radioisotopes from liquid phase. J. Mol. Liq. 2019;290:111237. doi: 10.1016/j.molliq.2019.111237. - DOI
-
- Hashem A., Sanousy M.A., Mohamed L.A., Okoye P.U., Hameed B.H. Natural and Low-Cost P. turgidum for Efficient Adsorption of Hg(II) Ions from Contaminated Solution: Isotherms and Kinetics Studies. J. Polym. Environ. 2021;29:304–312. doi: 10.1007/s10924-020-01879-5. - DOI
-
- Maamoun I., Eljamal R., Falyouna O., Bensaida K., Sugihara Y., Eljamal O. Insights into kinetics, isotherms and thermodynamics of phosphorus sorption onto nanoscale zero-valent iron. J. Mol. Liq. 2021;328:115402. doi: 10.1016/j.molliq.2021.115402. - DOI
-
- Lagergren S.K. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898;24:1–39.
-
- Jasper E.E., Ajibola V.O., Onwuka J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020;10:132. doi: 10.1007/s13201-020-01218-y. - DOI
-
- Hashem A., Badawy S.M., Farag S., Mohamed L.A., Fletcher A.J., Taha G.M. Non-linear adsorption characteristics of modified pine wood sawdust optimised for adsorption of Cd(II) from aqueous systems. J. Environ. Chem. Eng. 2020;8:103966. doi: 10.1016/j.jece.2020.103966. - DOI
-
- Chatterjee R., Majumder C. Modelling of adsorption process in industrial wastewater treatment—A review. J. Indian Chem. Soc. 2019;96:499–506. doi: 10.5281/zenodo.5637964. - DOI
-
- Pholosi A., Naidoo E.B., Ofomaja A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. S. Afr. J. Chem. Eng. 2020;32:39–55. doi: 10.1016/j.sajce.2020.01.005. - DOI
-
- McKay G., Poots V.J.P. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 1980;30:279–292. doi: 10.1002/jctb.503300134. - DOI
-
- Weber W.J., Morris J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963;89:31–59. doi: 10.1061/JSEDAI.0000430. - DOI
-
- Khandaker S., Toyohara Y., Saha G.C., Awual M.R., Kuba T. Development of synthetic zeolites from bio-slag for cesium adsorption: Kinetic, isotherm and thermodynamic studies. J. Water Process. Eng. 2020;33:101055. doi: 10.1016/j.jwpe.2019.101055. - DOI
-
- Benmessaoud A., Nibou D., Mekatel E.H., Amokrane S. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020;39:153–171. doi: 10.30492/ijcce.2019.35116. - DOI
-
- Mahmoud M.E., Saad E.A., El-Khatib A.M., Soliman M.A., Allam E.A. Adsorptive removal of radioactive isotopes of cobalt and zinc from water and radioactive wastewater using TiO2/Ag2O nanoadsorbents. Prog. Nucl. Energy. 2018;106:51–63. doi: 10.1016/j.pnucene.2018.02.021. - DOI
-
- Luo W., Chen J., Lin H., Ye X. Biomass base membrane with phenol hydroxy-amino group for ultraselective adsorption of radioactive Co(II) in trace concentration. Sep. Purif. Technol. 2021;272:118878. doi: 10.1016/j.seppur.2021.118878. - DOI
-
- Awual M.R., Khraisheh M., Alharthi N.H., Luqman M., Islam A., Karim M.R., Rahman M.M., Khaleque M.A. Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. 2018;343:118–127. doi: 10.1016/j.cej.2018.02.116. - DOI
-
- Zsigmondy R. Über amikroskopische Goldkeime. I. Z. Für Phys. Chem. 1906;56U:65–76. doi: 10.1515/zpch-1906-5605. - DOI
-
- Langmuir I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918;40:1361–1403. doi: 10.1021/ja02242a004. - DOI
-
- Temkin M., Pyzhev V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS. 1940;12:217–225.
-
- Chen T., Wang Q., Lyu J., Bai P., Guo X. Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2020;231:115930. doi: 10.1016/j.seppur.2019.115930. - DOI
-
- Marcu C., Balla A., Balázs J.Z.S., Lar C. Adsorption Isotherms and Thermodynamics for Chromium(VI) Using an Anion Exchange Resin. Anal. Lett. 2021;54:1783–1793. doi: 10.1080/00032719.2020.1825464. - DOI