Post-Marketing Experience of Edaravone in Amyotrophic Lateral Sclerosis: A Clinical Perspective and Comparison With the Clinical Trials of the Drug
Affiliations
Affiliations
- 1Neurology, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA.
- 2Research, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA.
- 3Hospital Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA.
- 4Internal Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA.
- 5Emergency Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA.
- 6Emergency Department, The Kidney Center, Karachi, PAK.
Abstract
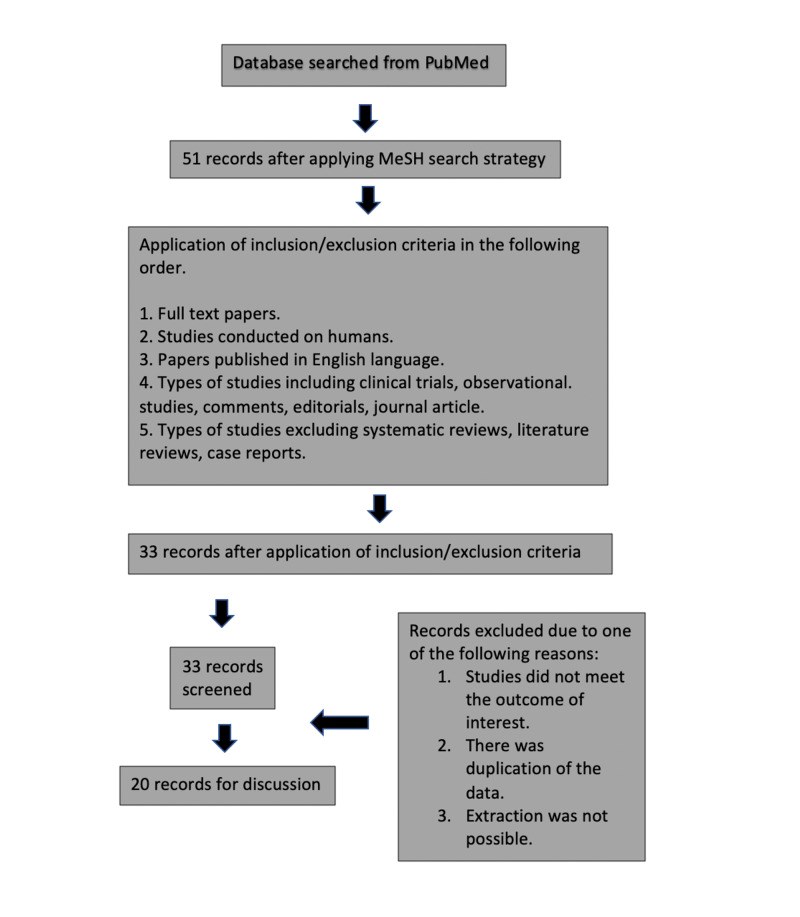
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative disease that affects the upper and lower motor neurons. Currently, the only treatment for ALS is riluzole, which only has a limited effect on increasing survival from 3 to 6 months. New therapies are needed in the clinical setting for ALS. We aim to compare and contrast the clinical trials of edaravone and the post-marketing experience of the drug during this study. For the method, a search strategy was made using PubMed with the search terms "Amyotrophic lateral sclerosis" (MeSH) and "Edaravone" (MeSH). For inclusion criteria, we used full papers, studies involving humans, and studies published in the English language. We exclude meta-analyses, literature reviews, systematic reviews, studies involving animals, and studies not published in English. After close examination, 20 papers were used for the discussion in this review. The clinical trials showed efficacy in patients in reducing the revised ALS functional rating scale (ALSFRS-R) in patients with early ALS with selective criteria. We documented edaravone's post-marketing experience in six countries: Kuwait, South Korea, Argentina, United States, Israel, and Italy. During the study we analyzed, the forced vital capacity (FVC) and ALSFRS-R scored, together with edaravone's safety in the clinical trials and post-marketing experience. Edaravone seems to be more effective in Asia, where the ALSFRS-R scores and the FVC decline were similar to the clinical trial results in Japan. Studies in Europe did not find the drug clinically useful. At the same time, studies in United States and Argentina were mainly descriptive, so more information is needed to evaluate the drug's efficacy in that part of the world. The drug was well-tolerated in all studies. In conclusion, more studies need to be done worldwide to carry out and clarify the effectiveness of edaravone in the clinical setting.
Keywords: amyotrophic lateral sclerosis; edaravone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
Brooks BR, Heiman-Patterson T, Wiedau-Pazos M, Liu S, Zhang J, Apple S.PLoS One. 2022 Jun 14;17(6):e0258614. doi: 10.1371/journal.pone.0258614. eCollection 2022.PMID: 35700157 Free PMC article. Clinical Trial.
Park JM, Park D, Kim HJ, Park JS.BMC Neurol. 2022 Jul 14;22(1):260. doi: 10.1186/s12883-022-02788-x.PMID: 35836136 Free PMC article.
WRITING GROUP ON BEHALF OF THE EDARAVONE (MCI-186) ALS 18 STUDY GROUP.Amyotroph Lateral Scler Frontotemporal Degener. 2017 Oct;18(sup1):40-48. doi: 10.1080/21678421.2017.1361441.PMID: 28872915 Clinical Trial.
Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis.
Sawada H.Expert Opin Pharmacother. 2017 May;18(7):735-738. doi: 10.1080/14656566.2017.1319937.PMID: 28406335 Review.
Edaravone and its clinical development for amyotrophic lateral sclerosis.
Takei K, Watanabe K, Yuki S, Akimoto M, Sakata T, Palumbo J.Amyotroph Lateral Scler Frontotemporal Degener. 2017 Oct;18(sup1):5-10. doi: 10.1080/21678421.2017.1353101.PMID: 28872907 Review.
Cited by
Anderson G.Int J Mol Sci. 2022 Dec 29;24(1):587. doi: 10.3390/ijms24010587.PMID: 36614029 Free PMC article. Review.
Use of Riluzole for the Treatment of Hereditary Ataxias: A Systematic Review.
Ayala IN, Aziz S, Argudo JM, Yepez M, Camacho M, Ojeda D, Aguirre AS, Oña S, Andrade AF, Vasudhar A, Moncayo JA, Hassen G, Ortiz JF, Tambo W.Brain Sci. 2022 Aug 5;12(8):1040. doi: 10.3390/brainsci12081040.PMID: 36009103 Free PMC article. Review.
Ethnical Disparities in Response to Edaravone in Patients With Amyotrophic Lateral Sclerosis.
Jayasinghe M, Jena R, Singhal M, Jain S, Karnakoti S, Silva MS, Kayani AMA.Cureus. 2022 Jun 15;14(6):e25960. doi: 10.7759/cureus.25960. eCollection 2022 Jun.PMID: 35855239 Free PMC article. Review.
Analysis of the US Safety Data for Edaravone (Radicava®) From the Third Year After Launch.
Genge A, Brooks BR, Oskarsson B, Kalin A, Ji M, Apple S, Bower L.Drugs R D. 2022 Sep;22(3):205-211. doi: 10.1007/s40268-022-00391-6. Epub 2022 Jun 20.PMID: 35723868 Free PMC article.
Brooks BR, Heiman-Patterson T, Wiedau-Pazos M, Liu S, Zhang J, Apple S.PLoS One. 2022 Jun 14;17(6):e0258614. doi: 10.1371/journal.pone.0258614. eCollection 2022.PMID: 35700157 Free PMC article. Clinical Trial.
KMEL References
References
-
- The epidemiology of amyotrophic lateral sclerosis. Talbott EO, Malek AM, Lacomis D. Handb Clin Neurol. 2016;138:225–238. - PubMed
-
- Amyotrophic lateral sclerosis: an update for 2018. Oskarsson B, Gendron TF, Staff NP. Mayo Clin Proc. 2018;93:1617–1628. - PubMed
-
- Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Miller RG, Mitchell JD, Lyon M, Moore DH. Cochrane Database Syst Rev. 2002:0. - PubMed
-
- Disease-modifying and symptomatic treatment of amyotrophic lateral sclerosis. Dorst J, Ludolph AC, Huebers A. Ther Adv Neurol Disord. 2017;11:1–16.
-
- Edaravone for amyotrophic lateral sclerosis: more evidence for long-term benefit. Distad BJ, Weiss MD. Muscle Nerve. 2020;61:129–130. - PubMed
-
- Edaravone: a new treatment for ALS on the horizon? Hardiman O, van den Berg LH. Lancet Neurol. 2017;16:490–491. - PubMed
-
- Post-hoc analysis of randomized, placebo-controlled, double-blind study (MCI186-19) of edaravone (MCI-186) in amyotrophic lateral sclerosis. Takei K, Takahashi F, Liu S, Tsuda K, Palumbo J. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:49–54. - PubMed
-
- Update on amyotrophic lateral sclerosis genetics. Brenner D, Weishaupt JH. Curr Opin Neurol. 2019;32:735–739. - PubMed
-
- Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study) Yoshino H, Kimura A. Amyotroph Lateral Scler. 2006;7:241–245. - PubMed
-
- Safety and efficacy of edaravone in well-defined patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled trial. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Lancet Neurol. 2017;16:505–512. - PubMed
-
- Post-hoc analysis of MCI186-17, the extension study to MCI186-16, the confirmatory double-blind, parallel-group, placebo-controlled study of edaravone in amyotrophic lateral sclerosis. Takahashi F, Takei K, Tsuda K, Palumbo J. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:32–39. - PubMed
-
- Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: grade 3, requiring assistance for eating, excretion or ambulation) The Writing Group on Behalf of the Edaravone (MCI-186) ALS 18 Study Group. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:40–48. - PubMed
-
- Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Writing Group on Behalf of the Edaravone (MCI-186) ALS 17 Study Group. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:20–31. - PubMed
-
- Post-hoc analysis of open-label extension period of study MCI186-19 in amyotrophic lateral sclerosis. Takei K, Tsuda K, Takahashi F, Palumbo J. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:64–70. - PubMed
-
- A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Edaravone (MCI-186) ALS 16 Study Group. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:11–19. - PubMed
-
- Radicava (edaravone) for amyotrophic lateral sclerosis: US experience at 1 year after launch. Jackson C, Heiman-Patterson T, Kittrell P, et al. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:605–610. - PubMed
-
- Logistics and safety of edaravone treatment for amyotrophic lateral sclerosis: experience in Argentina [IN PRESS] Quarracino C, Bendersky M, Rey R. Acta Neurol Belg. 2020 - PubMed
-
- Evaluation of clinical outcome and safety profile of edaravone in treatment of amyotrophic lateral sclerosis: a 72-week single-center experience [IN PRESS] Ismail II, Massoud F, Kamel WA, Al-Hashel JY. Acta Neurol Belg. 2020 - PubMed
-
- Early post-marketing experience with edaravone in an unselected group of patients with ALS. Abraham A, Nefussy B, Fainmesser Y, et al. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:260–263. - PubMed
-
- Safety and efficacy of edaravone compared to historical controls in patients with amyotrophic lateral sclerosis from North-Eastern Italy. Fortuna A, Gizzi M, Bello L, et al. J Neurol Sci. 2019;404:47–51. - PubMed
-
- The Italian multicenter experience with edaravone in amyotrophic lateral sclerosis [IN PRESS] Lunetta C, Moglia C, Lizio A, et al. J Neurol. 2020 - PubMed
-
- Clinical significance in the change of decline in ALSFRS-R. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Amyotroph Lateral Scler. 2010;11:178–180. - PubMed