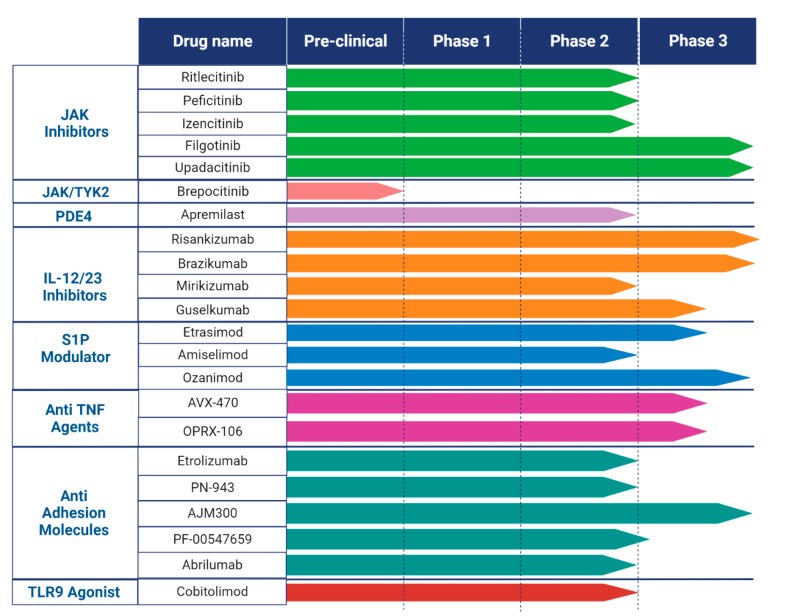
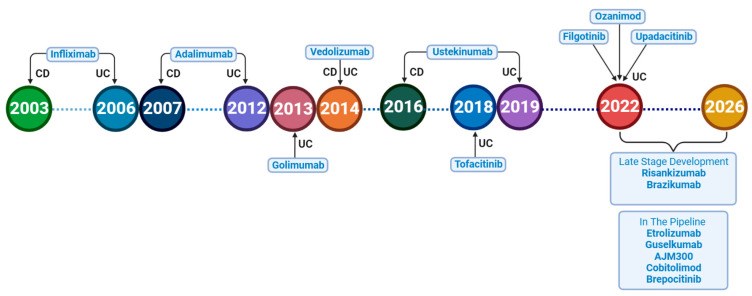
Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review
Affiliations
Affiliations
- Department of Internal Medicine, Mubarak Al-Kabeer Hospital, Kuwait University, Jabriya 46300, Kuwait.
- IBD Edinburgh Unit, Department of Medicine, Western General Hospital, Edinburgh EH4 2XU, UK.
- Gastroenterology Department, Reina Sofía University Hospital, 30003 Córdoba, Spain.
Abstract
Crohn's disease (CD) and ulcerative colitis (UC), known as inflammatory bowel diseases (IBD), are characterized by chronic inflammation of the gastrointestinal tract. Over the last two decades, numerous medications have been developed and repurposed to induce and maintain remission in IBD patients. Despite the approval of multiple drugs, the major recurring issues continue to be primary non-response and secondary loss of response, as well as short- and long-term adverse events. Most clinical trials show percentages of response under 60%, possibly as a consequence of strict inclusion criteria and definitions of response. That is why these percentages appear to be more optimistic in real-life studies. A therapeutic ceiling has been used as a term to define this invisible bar that has not been crossed by any drug yet. This review highlights novel therapeutic target agents in phases II and III of development, such as sphingosine-1-phosphate receptor modulators, selective Janus kinase inhibitors, anti-interleukins, and other small molecules that are currently under research until 1 January 2023. Emerging treatments for CD and UC that have just received approval or are undergoing phase III clinical trials are also discussed in this review.
Keywords: Crohn’s disease; IBD; Janus kinase inhibitor; interleukins; small molecules; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
The Impact of Inflammatory Bowel Disease in Canada 2018: Children and Adolescents with IBD.
Carroll MW, Kuenzig ME, Mack DR, Otley AR, Griffiths AM, Kaplan GG, Bernstein CN, Bitton A, Murthy SK, Nguyen GC, Lee K, Cooke-Lauder J, Benchimol EI.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S49-S67. doi: 10.1093/jcag/gwy056. Epub 2018 Nov 2.PMID: 31294385 Free PMC article.
Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis.
Davies SC, Hussein IM, Nguyen TM, Parker CE, Khanna R, Jairath V.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD012381. doi: 10.1002/14651858.CD012381.pub2.PMID: 31984480 Free PMC article.
Kuenzig ME, Benchimol EI, Lee L, Targownik LE, Singh H, Kaplan GG, Bernstein CN, Bitton A, Nguyen GC, Lee K, Cooke-Lauder J, Murthy SK.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S17-S33. doi: 10.1093/jcag/gwy055. Epub 2018 Nov 2.PMID: 31294382 Free PMC article.
Sinopoulou V, Gordon M, Akobeng AK, Gasparetto M, Sammaan M, Vasiliou J, Dovey TM.Cochrane Database Syst Rev. 2021 Nov 29;11(11):CD013531. doi: 10.1002/14651858.CD013531.pub2.PMID: 34844288 Free PMC article. Review.
Li Y, Chen J, Bolinger AA, Chen H, Liu Z, Cong Y, Brasier AR, Pinchuk IV, Tian B, Zhou J.Inflamm Bowel Dis. 2021 Nov 15;27(Suppl 2):S38-S62. doi: 10.1093/ibd/izab190.PMID: 34791293 Review.
Cited by
Alharbi TS, Alshammari ZS, Alanzi ZN, Althobaiti F, Elewa MAF, Hashem KS, Al-Gayyar MMH.Mol Cell Biochem. 2023 Apr 21. doi: 10.1007/s11010-023-04746-8. Online ahead of print.PMID: 37084167
KMEL References
References
-
- de Souza H.S.P., Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nature Reviews. Gastroenterol. Hepatol. 2015;13:13–27. - PubMed
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. - DOI - PubMed
-
- Peyrin-Biroulet L., Sandborn W., Sands B.E., Reinisch W., Bemelman W., Bryant R.V., D’Haens G., Dotan I., Dubinsky M., Feagan B., et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. - DOI - PubMed
-
- Turner D., Ricciuto A., Lewis A., D’Amico F., Dhaliwal J., Griffiths A.M., Bettenworth D., Sandborn W.J., Sands B.E., Reinisch W., et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
-
- Eftychi C., Schwarzer R., Vlantis K., Wachsmuth L., Basic M., Wagle P., Neurath M.F., Becker C., Bleich A., Pasparakis M., et al. Temporally Distinct Functions of the Cytokines IL-12 and IL-23 Drive Chronic Colon Inflammation in Response to Intestinal Barrier Impairment. Immunity. 2019;51:367–380.e4. doi: 10.1016/j.immuni.2019.06.008. - DOI - PubMed
-
- Leach M.W., Rennick Natalie JDavidson D.M., Hudak S.A., Lesley R.E. IL-10-Deficient Mice Sustaining the Chronic Phase of Colitis in, Plays a Major Role in γ IL-12, But Not IFN. 2022. [(accessed on 7 January 2023)]. Available online: http://www.jimmunol.org/content/161/6/3143. - PubMed
-
- Rodig S.J., Meraz M.A., White J.M., Lampe P.A., Riley J.K., Arthur C.D., King K.L., Sheehan K.C., Yin L., Pennica D., et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/S0092-8674(00)81166-6. - DOI - PubMed
-
- Sphingosine-1 Phosphate Receptor Modulators: The Next Wave of Oral Therapies in Inflammatory Bowel Disease–Gastroenterology & Hepatology. [(accessed on 9 January 2023)]. Available online: https://www.gastroenterologyandhepatology.net/archives/may-2022/sphingos... - PMC - PubMed
-
- D’Haens G., Panaccione R., Baert F., Bossuyt P., Colombel J.F., Danese S., Dubinsky M., Feagan B.G., Hisamatsu T., Lim A., et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015–2030. doi: 10.1016/S0140-6736(22)00467-6. - DOI - PubMed
-
- Feagan B.G., Sandborn W.J., D’Haens G., Panés J., Kaser A., Ferrante M., Louis E., Franchimont D., Dewit O., Seidler U., et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: A randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709. doi: 10.1016/S0140-6736(17)30570-6. - DOI - PubMed
-
- Ferrante M., Panaccione R., Baert F., Bossuyt P., Colombel J.F., Danese S., Dubinsky M., Feagan B.G., Hisamatsu T., Lim A., et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: Results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399:2031–2046. doi: 10.1016/S0140-6736(22)00466-4. - DOI - PubMed
-
- Feagan B.G., Panés J., Ferrante M., Kaser A., D’Haens G.R., Sandborn W.J., Louis E., Neurath M.F., Franchimont D., Dewit O., et al. Risankizumab in patients with moderate to severe Crohn’s disease: An open-label extension study. Lancet Gastroenterol. Hepatol. 2018;3:671–680. doi: 10.1016/S2468-1253(18)30233-4. - DOI - PubMed
-
- Ferrante M., Feagan B.G., Panés J., Baert F., Louis E., Dewit O., Kaser A., Duan W.R., Pang Y., Lee W.J., et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohns Colitis. 2021;15:2001–2010. doi: 10.1093/ecco-jcc/jjab093. - DOI - PMC - PubMed
-
- Long-Term Safety in the Open-Label Period of a Phase 2a Stud..: Official Journal of the American College of Gastroenterology|ACG. [(accessed on 7 October 2022)]. Available online: https://journals.lww.com/ajg/fulltext/2018/10001/long_term_safety_in_the....
-
- Janssen Announces u.s. Fda Approval of Tremfyatm (Guselkumab) for the Treatment of Moderate to Severe Plaque Psoriasis|Johnson & Johnson. [(accessed on 11 January 2023)]. Available online: https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda....
-
- Cdjjlgaoaa S. The efficacy and safety OF guselkumab induction therapy IN patients with moderately to severely active CROHN’S disease: Week 12 interim analyses from the phase 2 GALAXI 1 study. United Eur. Gastroenterol. J. 2020;8:64.
-
- Sandborn W.J., D’Haens G.R., Reinisch W., Panés J., Chan D., Gonzalez S., Weisel K., Germinaro M., Frustaci M.E., Yang Z., et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results From the Phase 2 GALAXI-1 Study. Gastroenterology. 2022;162:1650–1664.e8. doi: 10.1053/j.gastro.2022.01.047. - DOI - PubMed
-
- Sandborn W.J., Ferrante M., Bhandari B.R., Berliba E., Feagan B.G., Hibi T., Tuttle J.L., Klekotka P., Friedrich S., Durante M., et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Ulcerative Colitis. Gastroenterology. 2020;158:537–549.e10. doi: 10.1053/j.gastro.2019.08.043. - DOI - PubMed
-
- Sandborn W.J., Ferrante M., Bhandari B.R., Berliba E., Hibi T., D’Haens G.R., Tuttle J.L., Klekotka P., Friedrich S., Durante M., et al. Efficacy and Safety of Continued Treatment With Mirikizumab in a Phase 2 Trial of Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2022;20:105–115.e14. doi: 10.1016/j.cgh.2020.09.028. - DOI - PubMed
-
- Harris M.S., Hartman D., Lemos B.R., Erlich E.C., Spence S., Kennedy S., Ptak T., Pruitt R., Vermeire S., Fox B.S. AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J. Crohns Colitis. 2016;10:631–640. doi: 10.1093/ecco-jcc/jjw036. - DOI - PubMed
-
- Almon E., Shaaltiel Y., Sbeit W., Fich A., Schwartz D., Waterman M., Szlaifer M., Reuveni H., Amit-Cohen B.C., Alon S., et al. Novel Orally Administered Recombinant Anti-TNF Alpha Fusion Protein for the Treatment of Ulcerative Colitis: Results From a Phase 2a Clinical Trial. J. Clin. Gastroenterol. 2021;55:134. doi: 10.1097/MCG.0000000000001314. - DOI - PMC - PubMed
-
- A Clinical Trial to Compare Etrolizumab with Placebo and Adalimumab in Patients with Moderate to Severe Ulcerative Colitis Who Have not Received Treatment with Tumour Necrosis Factor Inhibitors (Hibiscus I) [(accessed on 3 January 2023)]. Available online: https://forpatients.roche.com/en/trials/autoimmune-disorder/ulcerative-c....
-
- A Clinical Trial to Compare Etrolizumab with Placebo and Adalimumab in Patients with Moderate to Severe Ulcerative Colitis Who Have not Received Treatment with Tumour Necrosis Factor Inhibitors (Hibiscus II) [(accessed on 3 January 2023)]. Available online: https://genentech-clinicaltrials.com/en/trials/autoimmune-disorder/ulcer....
-
- Danese S., Colombel J.F., Lukas M., Gisbert J.P., D’Haens G., Hayee B., Panaccione R., Kim H.S., Reinisch W., Tyrrell H., et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): A randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 2022;7:118–127. doi: 10.1016/S2468-1253(21)00294-6. - DOI - PubMed
-
- Sandborn W.J., Vermeire S., Tyrrell H., Hassanali A., Lacey S., Tole S., Tatro A.R., Etrolizumab Global Steering Committee Etrolizumab for the Treatment of Ulcerative Colitis and Crohn’s Disease: An Overview of the Phase 3 Clinical Program. Adv. Ther. 2020;37:3417–3431. doi: 10.1007/s12325-020-01366-2. - DOI - PMC - PubMed
-
- Dai B., Hackney J.A., Ichikawa R., Nguyen A., Elstrott J., Orozco L.D., Sun K.H., Modrusan Z., Gogineni A., Scherl A., et al. Dual targeting of lymphocyte homing and retention through α4β7 and αEβ7 inhibition in inflammatory bowel disease. Cell Rep. Med. 2021;2:10038. doi: 10.1016/j.xcrm.2021.100381. - DOI - PMC - PubMed
-
- Modi N.B., Cheng X., Mattheakis L., Hwang C.C., Nawabi R., Liu D., Gupta S. Single- and Multiple-Dose Pharmacokinetics and Pharmacodynamics of PN-943, a Gastrointestinal-Restricted Oral Peptide Antagonist of α4β7, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2021;10:1263–1278. doi: 10.1002/cpdd.946. - DOI - PMC - PubMed
-
- Mattheakis L., Bhandari A., Bai L., Zemede G., Tran V., Celino H., Frederick B., Zhao L., Dogra M., Lister H., et al. P-126 PTG-100, An Oral Peptide Antagonist of Integrin α4β7 that Alters Trafficking of Gut Homing T Cells in Preclinical Animal Models. Inflamm. Bowel Dis. 2016;22((Suppl. 1)):S48. doi: 10.1097/01.MIB.0000480232.55276.b3. - DOI
-
- Mattheakis L., Fosser C., Saralaya R., Horsch K., Rao N., Bai L., Zhao L., Annamalai T., Liu D. P113 Model based predictions of the PTG-100 pharmacodynamic responses in ulcerative colitis patients. J. Crohns Colitis. 2017;11((Suppl. 1)):S132–S133. doi: 10.1093/ecco-jcc/jjx002.239. - DOI
-
- PN-943 in Adults With Moderate to Severe Active Ulcerative Colitis (UC) [(accessed on 13 October 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04504383.
-
- Sandborn W.J., Lee S.D., Tarabar D., Louis E., Klopocka M., Klaus J., Reinisch W., Hébuterne X., Park D.I., Schreiber S., et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: Report of the OPERA study. Gut. 2018;67:1824–1835. doi: 10.1136/gutjnl-2016-313457. - DOI - PMC - PubMed
-
- Vermeire S., Sandborn W.J., Danese S., Hébuterne X., Salzberg B.A., Klopocka M., Tarabar D., Vanasek T., Greguš M., Hellstern P.A., et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135–144. doi: 10.1016/S0140-6736(17)30930-3. - DOI - PubMed
-
- Sandborn W.J., Cyrille M., Berner Hansen M., Feagan B.G., Loftus E.V., Vermeire S., Cruz M.L., Mo M., Sullivan B.A., Reinisch W. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J. Crohns Colitis. 2017;11((Suppl. 1)):S22–S23. doi: 10.1093/ecco-jcc/jjx002.034. - DOI
-
- Sandborn W.J., Cyrille M., Hansen M.B., Feagan B.G., Loftus E., Rogler G., Vermeire S., Cruz M.L., Yang J., Boedigheimer M.J., et al. Efficacy and Safety of Abrilumab in a Randomized, Placebo-Controlled Trial for Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2019;156:946–957.e18. doi: 10.1053/j.gastro.2018.11.035. - DOI - PubMed
-
- Sugiura T., Kageyama S., Andou A., Miyazawa T., Ejima C., Nakayama A., Dohi T., Eda H. Oral treatment with a novel small molecule alpha 4 integrin antagonist, AJM300, prevents the development of experimental colitis in mice. J. Crohns Colitis. 2013;7:e533–e542. doi: 10.1016/j.crohns.2013.03.014. - DOI - PubMed
-
- Yoshimura N., Watanabe M., Motoya S., Tominaga K., Matsuoka K., Iwakiri R., Watanabe K., Hibi T., AJM300 Study Group Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology. 2015;149:1775–1783.e2. doi: 10.1053/j.gastro.2015.08.044. - DOI - PubMed
-
- Takazoe M., Watanabe M., Kawaguchi T., Matsumoto T., Oshitani N., Hiwatashi N., Toshifumi H. S1066 Oral Alpha-4 Integrin Inhibitor (AJM300) in Patients with Active Crohn’s Disease—A Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2009;5((Suppl. 1)):A-181. doi: 10.1016/S0016-5085(09)60816-7. - DOI
-
- Lassiter G., Melancon C., Rooney T., Murat A.M., Kaye J.S., Kaye A.M., Kaye R.J., Cornett E.M., Kaye A.D., Shah R.J., et al. Ozanimod to Treat Relapsing Forms of Multiple Sclerosis: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Neurol. Int. 2020;12:89–108. doi: 10.3390/neurolint12030016. - DOI - PMC - PubMed
-
- Sandborn W.J., Feagan B.G., Hanauer S., Vermeire S., Ghosh S., Liu W.J., Petersen A., Charles L., Huang V., Usiskin K., et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohns Colitis. 2021;15:1120–1129. doi: 10.1093/ecco-jcc/jjab012. - DOI - PMC - PubMed
-
- Dgwshsjigs S. Ozanimod As Induction Therapy in Moderate-To-Severe Ulcerative Colitis: Results from The Phase 3. Randomized, Double-Blind, Placebo-Controlled True North Study. United Eur. Gastroenterol. J. 2020;8:3.
-
- Feagan B.G., Sandborn W.J., Danese S., Wolf D.C., Liu W.J., Hua S.Y., Minton N., Olson A., D’Haens G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020;5:819–828. doi: 10.1016/S2468-1253(20)30188-6. - DOI - PubMed
-
- Sandborn W.J., Peyrin-Biroulet L., Zhang J., Chiorean M., Vermeire S., Lee S.D., Kühbacher T., Yacyshyn B., Cabell C.H., Naik S.U., et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology. 2020;158:550–561.e9. doi: 10.1053/j.gastro.2019.10.035. - DOI - PubMed
-
- D’Haens G., Danese S., Davies M., Watanabe M., Hibi T. A phase II, Multicentre, Randomised, Double-Blind, Placebo-controlled Study to Evaluate Safety, Tolerability, and Efficacy of Amiselimod in Patients with Moderate to Severe Active Crohn’s Disease. J. Crohns Colitis. 2022;16:746–756. doi: 10.1093/ecco-jcc/jjab201. - DOI - PubMed
-
- Papp K., Reich K., Leonardi C.L., Kircik L., Chimenti S., Langley R.G.B., Hu C., Stevens R.M., Day R.M., Gordon K.B., et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM) 1) J. Am. Acad. Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. - DOI - PubMed
-
- Danese S., Neurath M.F., Kopoń A., Zakko S.F., Simmons T.C., Fogel R., Siegel C.A., Panaccione R., Zhan X., Usiskin K., et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients With Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020;18:2526–2534.e9. doi: 10.1016/j.cgh.2019.12.032. - DOI - PubMed
-
- Panés J., Vermeire S., Lindsay J.O., Sands B.E., Su C., Friedman G., Zhang H., Yarlas A., Bayliss M., Maher S., et al. Tofacitinib in Patients with Ulcerative Colitis: Health-Related Quality of Life in Phase 3 Randomised Controlled Induction and Maintenance Studies. J. Crohns Colitis. 2019;13:139–140. doi: 10.1093/ecco-jcc/jjy135. - DOI - PMC - PubMed
-
- Sandborn W.J., Peyrin-Biroulet L., Sharara A.I., Su C., Modesto I., Mundayat R., Gunay L.M., Salese L., Sands B.E. Efficacy and Safety of Tofacitinib in Ulcerative Colitis Based on Prior Tumor Necrosis Factor Inhibitor Failure Status. Clin. Gastroenterol. Hepatol. 2022;20:591–601.e8. doi: 10.1016/j.cgh.2021.02.043. - DOI - PubMed
-
- Vermeire S., Schreiber S., Petryka R., Kuehbacher T., Hebuterne X., Roblin X., Klopocka M., Goldis A., Wisniewska-Jarosinska M., Baranovsky A., et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. doi: 10.1016/S0140-6736(16)32537-5. - DOI - PubMed
-
- Peyrin-Biroulet L., Loftus E., Danese S., Vermeire S., Sandborn W.J., Fogel R., Nijhawan S., Kempinski R., Filip R., Hospodarskyy I., et al. A17 efficacy and safety of filgotinib as maintenance therapy for patients with moderately to severely active ulcerative colitis: Results from the phase 2B/3 selection study. J. Can. Assoc. Gastroenterol. 2021;4((Suppl. 1)):21–23. doi: 10.1093/jcag/gwab002.016. - DOI - PubMed
-
- Danese S., Vermeire S., Zhou W., Pangan A.L., Siffledeen J., Greenbloom S., Hébuterne X., D’Haens G., Nakase H., Panés J., et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. doi: 10.1016/S0140-6736(22)00581-5. - DOI - PubMed
-
- Sandborn W.J., Feagan B.G., Loftus E.V., van Assche G., D’Haens G., Schreiber S., Colombel J.F., Lewis J.D., Ghosh S., Peyrin-Biroulet L., et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology. 2020;158:2123–2138.e8. doi: 10.1053/j.gastro.2020.01.047. - DOI - PubMed
-
- Sands B.E., Sandborn W.J., Feagan B.G., Lichtenstein G.R., Zhang H., Strauss R., Szapary P., Johanns J., Panes J., Vermeire S., et al. Peficitinib, an Oral Janus Kinase Inhibitor, in Moderate-to-severe Ulcerative Colitis: Results From a Randomised, Phase 2 Study. J. Crohns Colitis. 2018;12:1158–1169. doi: 10.1093/ecco-jcc/jjy085. - DOI - PubMed
-
- Beattie D.T., Pulido-Rios M.T., Shen F., Ho M., Situ E., Tsuruda P.R., Brassil P., Kleinschek M., Hegde S. Intestinally-restricted Janus Kinase inhibition: A potential approach to maximize the therapeutic index in inflammatory bowel disease therapy. J. Inflamm. 2017;14:1–11. doi: 10.1186/s12950-017-0175-2. - DOI - PMC - PubMed
-
- Sandborn W.J., Nguyen D.D., Beattie D.T., Brassil P., Krey W., Woo J., Situ E., Sana R., Sandvik E., Pulido-Rios M., et al. Development of Gut-Selective Pan-Janus Kinase Inhibitor TD-1473 for Ulcerative Colitis: A Translational Medicine Programme. J. Crohns Colitis. 2020;14:1202–1213. doi: 10.1093/ecco-jcc/jjaa049. - DOI - PMC - PubMed
-
- Theravance’s Izencitinib Fails in Phase IIb Ulcerative Colitis Trial. [(accessed on 23 October 2022)]. Available online: https://www.clinicaltrialsarena.com/news/theravance-izencitinib-ulcerati...
-
- NCT03395184. Study To Evaluate The Efficacy and Safety of Oral PF-06651600 and PF 06700841 in Subjects with Moderate to Severe Crohn’s Disease. [(accessed on 8 January 2023)]. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01559550/.... - DOI
-
- Sandborn W., Danese S., Leszczyszyn J., Romatowski J., Altintas E., Peeva E., Vincent M., Reddy P., Banfield C., Banerjee A., et al. OP33 Oral ritlecitinib and brepocitinib in patients with Moderate to Severe Active Ulcerative Colitis: Data from the VIBRATO umbrella study. J. Crohns Colitis. 2021;15((Suppl. 1)):S030–S031. doi: 10.1093/ecco-jcc/jjab075.032. - DOI
-
- Mease P.J., Deodhar A.A., van der Heijde D., Behrens F., Kivitz A.J., Neal J., Kim J., Singhal S., Nowak M., Banerjee S. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815–822. doi: 10.1136/annrheumdis-2021-221664. - DOI - PMC - PubMed
-
- EUCTR2019-004878-26-NL. A Study of the Safety, Efficacy, and Biomarker Response of BMS-986165 in Participants with Moderate to Severe Ulcerative Colitis. [(accessed on 23 October 2022)]. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02256022/.... - DOI
-
- Bristol Myers Squibb-Bristol Myers Squibb Provides Update on Phase 2 Study of Deucravacitinib in Patients with Moderate to Severe Ulcerative Colitis. [(accessed on 23 October 2022)]. Available online: https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Provides-Upd....
-
- Atreya R., Bloom S., Scaldaferri F., Gerardi V., Admyre C., Karlsson Å., Knittel T., Kowalski J., Lukas M., Löfberg R., et al. Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis. J. Crohns Colitis. 2016;10:1294. doi: 10.1093/ecco-jcc/jjw103. - DOI - PMC - PubMed
-
- Atreya R., Peyrin-Biroulet L., Klymenko A., Augustyn M., Bakulin I., Slankamenac D., Miheller P., Gasbarrini A., Hébuterne X., Arnesson K., et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): A phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol. Hepatol. 2020;5:1063–1075. doi: 10.1016/S2468-1253(20)30301-0. - DOI - PubMed
-
- Study Record|Beta. [(accessed on 8 January 2023)]; Available online: https://beta.clinicaltrials.gov/study/NCT04985968.