The Effect of Platelet-Rich Plasma on Healing Time in Patients Following Pilonidal Sinus Surgery: A Systematic Review
Affiliations
Affiliations
- 1Department of General Surgery, Queen Elizabeth University Hospital, Glasgow, GBR.
- 2Department of General Surgery, Sabah Hospital, Kuwait, KWT.
- 3Department of Medical Education, University of Aberdeen, Aberdeen, GBR.
- 4Department of Emergency Medicine, University Hospital Ayr, Ayr, GBR.
- 5School of Medicine, University of Glasgow, Glasgow, GBR.
- 6Department of General Surgery, Victoria Hospital, Kirkcaldy, GBR.
Abstract
Background: Pilonidal disease (PD) is a debilitating condition characterised by the infection of subcutaneous tissue in the sacrococcygeal area. It is associated with a high risk of recurrence, pain, infection, and purulent discharge. The two main surgical methods of pilonidal sinus disease include excision with primary closure/flap repair or excision of the sinus with healing by secondary intent. Wounds left open to heal by secondary intent remain extremely common due to their association with reduced risk of recurrence, however, it is associated with prolonged healing times. This study aims to determine whether platelet-rich plasma (PRP) reduces healing time in patients post pilonidal sinus surgery with healing by secondary intent compared to simple wound dressings.
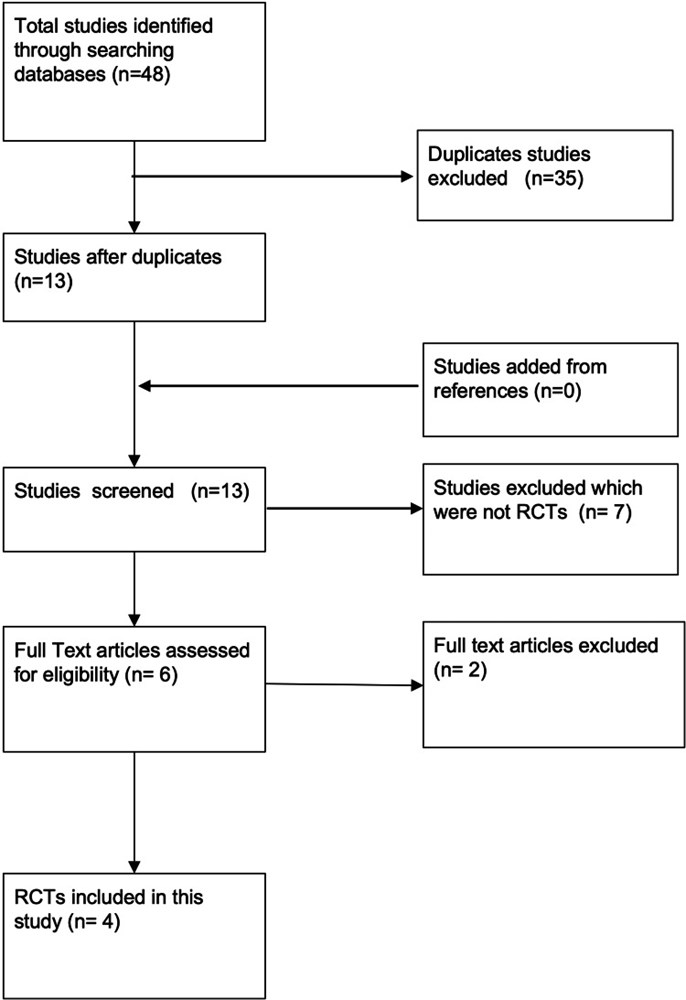
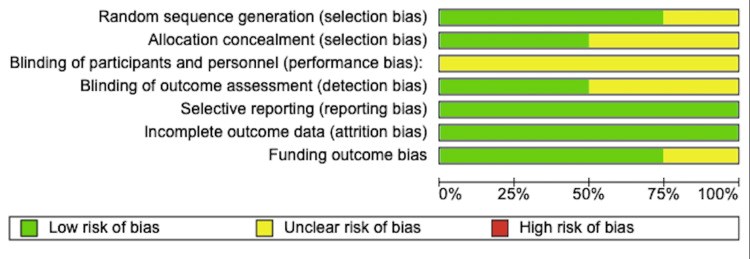
Method: Six databases were searched from their date of origin to May 30, 2022 for randomised control trials using predetermined inclusion and exclusion criteria. Only four papers were selected for review as per the Population, Intervention, Comparison, Outcomes and Study design (PICOS) criteria. Critical appraisal was carried out according to the Scottish Intercollegiate Guidelines Network Methodology Checklist for Randomised Control Trials and was assessed for risk of bias according to the Cochrane Handbook for Systematic Review of Interventions. The pooled effect size was calculated using the fixed-effect model. A homogeneity of pooled effect size for the studies was also found (Cochrane Q test, p-value = 0.97 I-square = 0.0%).
Result: Four studies (n = 336) were included in this review. Three of the four studies reported a statistically significant reduction in time taken in healing the wound. The mean difference between the intervention (PRP group) and the control group was 13.01 days, (95% CI 12.15-13.86 days, p < 0.00001). All of the included studies also reported a statistically significant reduction in time taken to return to work/activities of daily living in the treatment group compared to the control group (MD 9.68 days, 95% CI 9.16-10.21 days, p < 0.00001).
Conclusion: This study shows that PRP is effective in reducing healing time and is associated with a significantly shorter period taken to return to work/activities of daily living in patients post pilonidal sinus surgery, which was the primary and secondary outcome investigated in this systematic review, respectively. PRP should routinely be offered to patients undergoing excisional pilonidal sinus surgery for the aforementioned benefits.
Keywords: a systematic review; healing time; pilonidal sinus surgery; platelet-rich plasma/ prp; return to work.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
Brewer CF, Correia IFS, Miranda BH.World J Surg. 2022 Dec;46(12):2910-2918. doi: 10.1007/s00268-022-06711-w. Epub 2022 Sep 5.PMID: 36064868 Review.
Healing by primary versus secondary intention after surgical treatment for pilonidal sinus.
McCallum I, King PM, Bruce J.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006213. doi: 10.1002/14651858.CD006213.pub2.PMID: 17943897 Updated. Review.
Fibrin glue for pilonidal sinus disease.
Lund J, Tou S, Doleman B, Williams JP.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD011923. doi: 10.1002/14651858.CD011923.pub2.PMID: 28085995 Free PMC article. Review.
Gohar MM, Ali RF, Ismail KA, Ismail TA, Nosair NA.BMC Surg. 2020 Sep 22;20(1):212. doi: 10.1186/s12893-020-00865-x.PMID: 32962673 Free PMC article. Clinical Trial.
Healing by primary versus secondary intention after surgical treatment for pilonidal sinus.
Al-Khamis A, McCallum I, King PM, Bruce J.Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD006213. doi: 10.1002/14651858.CD006213.pub3.PMID: 20091589 Free PMC article. Review.
Cited by
Effects of Insular Cortex on Post-Stroke Dysphagia: A Systematic Review and Meta Analysis.
Qiao J, Wu Z, Cheng X, Ye Q, Dai M, Dai Y, Dou Z.Brain Sci. 2022 Oct 2;12(10):1334. doi: 10.3390/brainsci12101334.PMID: 36291268 Free PMC article. Review.
KMEL References
References
-
- Negative pressure wound therapy for recurrent pilonidal disease: a review of the literature. Farrell D, Murphy S. J Wound Ostomy Continence Nurs. 2011;38:373–378. - PubMed
-
- Global gender differences in pilonidal sinus disease: a random-effects meta-analysis. Luedi MM, Schober P, Stauffer VK, Diekmann M, Doll D. World J Surg. 2020;44:3702–3709. - PubMed
-
- Patient characteristics and symptoms in chronic pilonidal sinus disease. Søndenaa K, Andersen E, Nesvik I, Søreide JA. Int J Colorectal Dis. 1995;10:39–42. - PubMed
-
- Evaluation and management of pilonidal disease. Humphries AE, Duncan JE. Surg Clin North Am. 2010;90:113–124. - PubMed
-
- Pilo-nidal sinus. Hodges RM. Boston Med Surg J. 1880;103:485–486.
-
- Pathology of the postanal pilonidal sinus; its bearing on treatment. Patey DH, Scarff RW. Lancet. 1946;5:484–486. - PubMed
-
- Treatment of malignancy arising in pilonidal disease. De Bree E, Zoetmulder FAN, Christodoulakis M, et al. Ann Surg Oncol. 8:60–64. - PubMed
-
- Pilonidal sinus disease: early surgery and the limberg flap improve patient outcomes. Editorial Editorial. Adv Skin Wound Care. 2021;34:63. - PubMed
-
- Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Lindeboom JA, Mathura KR, Aartman IH, Kroon FH, Milstein DM, Ince C. Clin Oral Implants Res. 2007;18:133–139. - PubMed
-
- Platelet-rich plasma mixed-fat grafting: a reasonable prosurvival strategy for fat grafts? Serra-Mestre JM, Serra-Renom JM, Martinez L, Almadori A, D'Andrea F. Aesthetic Plast Surg. 2014;38:1041–1049. - PubMed
-
- Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: a single-arm clinical trial. Mohammadi MH, Molavi B, Mohammadi S, et al. Transfus Apher Sci. 2017;56:160–164. - PubMed
-
- Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Ferrara N, Henzel WJ. Biochem Biophys Res Commun. 1989;161:851–858. - PubMed
-
- Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: a randomized controlled parallel clinical trial. Mohammadi S, Nasiri S, Mohammadi MH, et al. Transfus Apher Sci. 2017;56:226–232. - PubMed
-
- The role of the platelet-rich plasma in accelerating the wound-healing process and recovery in patients being operated for pilonidal sinus disease: preliminary results. Spyridakis M, Christodoulidis G, Chatzitheofilou C, Symeonidis D, Tepetes K. World J Surg. 2009;33:1764–1769. - PubMed
-
- Scottish Intercollegiate Guidelines Network. SIGN methodology checklist for randomised controlled trials. [ May; 2022 ];https://www.sign.ac.uk/what-we-do/methodology/checklists/. 2020
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions, 2nd Edition. Chichester: John Wiley & Sons; 2019. - PubMed
![Figure 3. Forest plot of mean difference in time taken to heal between the platelet-rich plasma (PRP) group and control group Bahar et al. 2013 [16], Gohar et al. 2020 [17], Mohammadi et al. 2016 [18], Spyridakis et al. 2009 [19]](/Images/Figures/49_4957.jpeg)
![Figure 4. Forest plot of effect size in time to heal between the platelet-rich plasma (PRP) group and control group Bahar et al. 2013 [16], Gohar et al. 2020 [17], Mohammadi et al. 2016 [18], Spyridakis et al. 2009 [19]](/Images/Figures/49_5012.jpeg)