Tofacitinib for a Child with Refractory Steroid-Dependent Ulcerative Colitis: A Case Report and Review of the Literature
Affiliations
Affiliations
- Department of Internal Medicine, Farwaniya Hospital, Farwaniya, Kuwait.
- Department of Internal Medicine, Haya Alhabib Gastroenterology Center, Mubarak Alkabeer Hospital, Al-Jabreyah, Kuwait.
Abstract
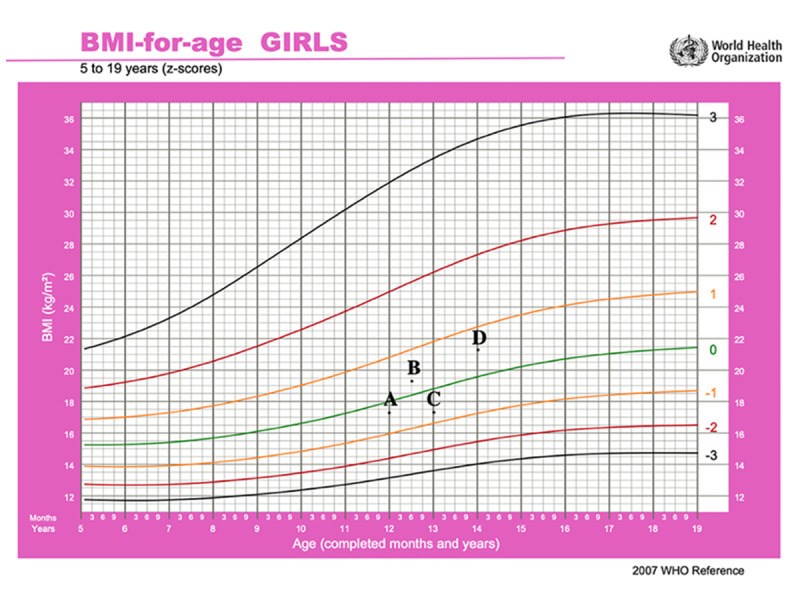
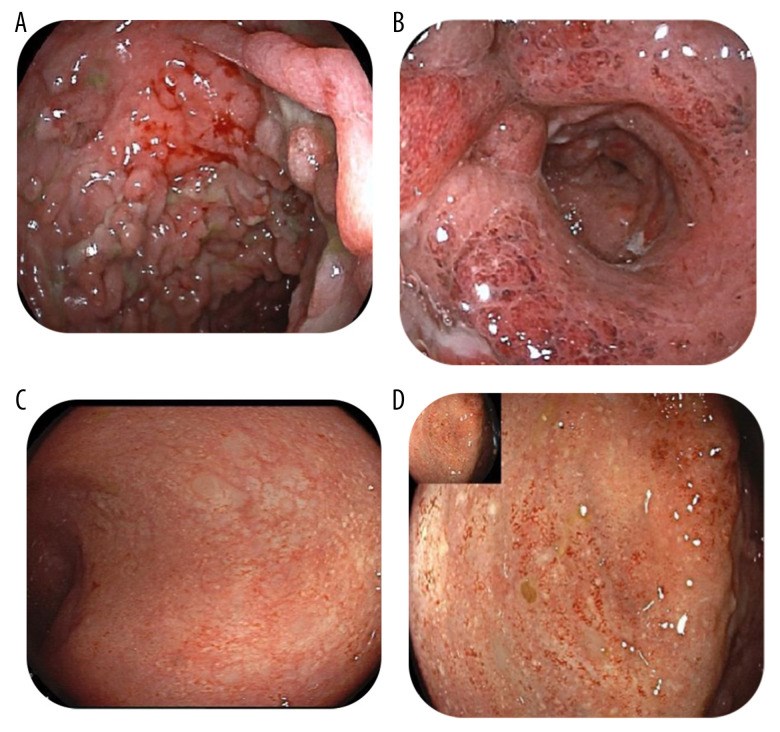
BACKGROUND Ulcerative colitis (UC) is a chronic autoimmune inflammatory disease of the colon that infrequently affects children. The disease requires immunosuppressive therapy to achieve remission and keep the disease in remission. Currently, many therapies are approved for use in pediatric patients with UC, including steroid, 5-aminosalicylic acid (5-ASA), azathioprine, and biologic therapy with anti-tumor necrosis factor (TNF) inhibitors. Despite their efficacy, many patients have refractory severe disease that fails therapy and may require surgical interventions. Recently, the small molecule Janus Kinase (JAK) inhibitor tofacitinib has been approved for moderate to severe UC that fails biologic therapy in adults. However, the safety and efficacy of this drug has not been tested in pediatric UC patients. CASE REPORT We describe a case of a 13-year-old girl with 2-year history of severe UC who had secondary loss response to both infliximab and adalimumab over 2 years, despite adequate trough serum drug levels and the concomitant use of azathioprine. She was also dependent on steroid to control her disease. Infectious work-ups were always negative for infectious organisms. She was then successfully treated with tofacitinib 5 mg orally twice daily. She went into complete clinical, endoscopic, and steroid-free remission. CONCLUSIONS This case report highlights the safety and efficacy of tofacitinib in pediatric patients with severe refractory UC, potentially avoiding proctocolectomy in this young patient population. Future research should study the role of tofacitinib in patients with moderate to severe UC in children.
Conflict of interest statement
Conflict of interest: None declared
Figures
Similar articles
Lair-Mehiri L, Stefanescu C, Vaysse T, Laharie D, Roblin X, Rosa I, Treton X, Abitbol V, Amiot A, Bouguen G, Dib N, Fumery M, Pariente B, Carbonnel F, Peyrin-Biroulet L, Simon M, Viennot S, Bouhnik Y.Dig Liver Dis. 2020 Mar;52(3):268-273. doi: 10.1016/j.dld.2019.10.003. Epub 2019 Nov 13.PMID: 31732444
Kakiuchi T, Yoshiura M.Medicine (Baltimore). 2022 Nov 11;101(45):e31757. doi: 10.1097/MD.0000000000031757.PMID: 36397383 Free PMC article.
Real-World Experience with Tofacitinib in IBD at a Tertiary Center.
Weisshof R, Aharoni Golan M, Sossenheimer PH, El Jurdi K, Ollech JE, Pekow J, Cohen RD, Sakuraba A, Dalal S, Rubin DT.Dig Dis Sci. 2019 Jul;64(7):1945-1951. doi: 10.1007/s10620-019-05492-y. Epub 2019 Feb 7.PMID: 30734234 Free PMC article.
Efficacy of tofacitinib treatment in ulcerative colitis.
Panés J, Gisbert JP.Gastroenterol Hepatol. 2019 Jun-Jul;42(6):403-412. doi: 10.1016/j.gastrohep.2019.03.002. Epub 2019 May 14.PMID: 31101342 Review. English, Spanish.
Varyani F, Argyriou K, Phillips F, Tsakiridou E, Moran GW.Drug Des Devel Ther. 2019 Dec 2;13:4091-4105. doi: 10.2147/DDDT.S182891. eCollection 2019.PMID: 31819376 Free PMC article. Review.
Cited by
Girard C, Dirks M, Deslandres C.JPGN Rep. 2022 Aug 16;3(3):e241. doi: 10.1097/PG9.0000000000000241. eCollection 2022 Aug.PMID: 37168636 Free PMC article.
KMEL References
References
-
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–29. - PubMed
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–23. - PubMed
-
- Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36. - PubMed
-
- Tseng B, Amighi A, Bradford K, et al. Tofacitinib response in juvenile idiopathic arthritis (JIA) and collagenous colitis. J Clin Rheumatol. 2016;22(8):446–48. - PubMed
-
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: Ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257–91. - PubMed
-
- Dolinger MT, Rolfes P, Phan BL, Dubinsky MC. Letter: Tofacitinib use for biologic-refractory paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50(8):966–67. - PubMed
-
- Dolinger MT, Spencer EA, Lai J, et al. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(8):1210–14. - PubMed
-
- Efficacy study of tofacitinib in pediatric JIA population – full text view – ClinicalTrials.gov. ClinicalTrials.gov. Published February 21, 2020. Accessed August 17, 2021. https://www.clinicaltrials.gov/ct2/show/NCT02592434.
-
- Evaluation of oral tofacitinib in children aged 2 to 17 years old suffering from moderate to severe ulcerative colitis – full text view – ClinicalTrials.gov. ClinicalTrials.gov. Published November 10, 2020. Accessed August 17, 2021. https://clinicaltrials.gov/ct2/show/NCT04624230.
-
- López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. 2021;44(1):39–48. - PubMed
-
- Serious heart events, cancer, blood clots for certain JAK inhibitors. U.S. Food and Drug Administration. Published 2021. Accessed October 2, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warn....
-
- Safety study of tofacitinib versus tumor necrosis factor (TNF) inhibitor in subjects with rheumatoid arthritis – full text view – ClinicalTrials.gov. Clinicaltrials.gov. Published 2021. Accessed October 2, 2021 https://clinicaltrials.gov/ct2/show/NCT02092467.