Oral Nystatin Prophylaxis for the Prevention of Fungal Colonization in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Affiliations
Affiliations
- Neonatology, King Fahad Medical City, Riyadh, SAU.
- General Practice, Faculty of Medicine, The Hashemite University, Zarqa, JOR.
- College of Medicine, Alfaisal University, Riyadh, SAU.
- Pediatrics, Jahra Hospital, Al Jahra, KWT.
- General Practice, Faculty of Medicine, Kuwait Institute for Medical Specializations, Kuwait City, KWT.
- Emergency Department, Adan Hospital, Al-Ahmadi, KWT.
- Pediatrics, Faculty of Medicine, Kuwait Institute for Medical Specializations, Kuwait City, KWT.
- College of Medicine, Imam Mohammad Ibn Saud Islamic University, Riyadh, SAU.
- Medicine, Jordan University of Science and Technology, Irbid, JOR.
- Pediatrics, King Khalid University Hospital, Riyadh, SAU.
- Internal Medicine, College of Graduate Health Sciences, The University of Tennessee Health Science Center, Memphis, USA.
Abstract
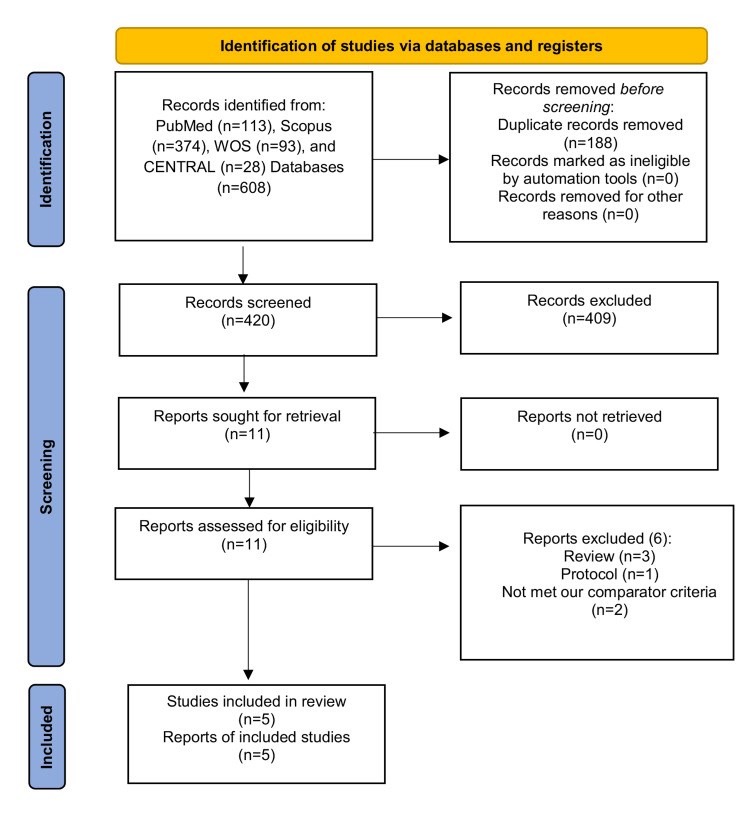
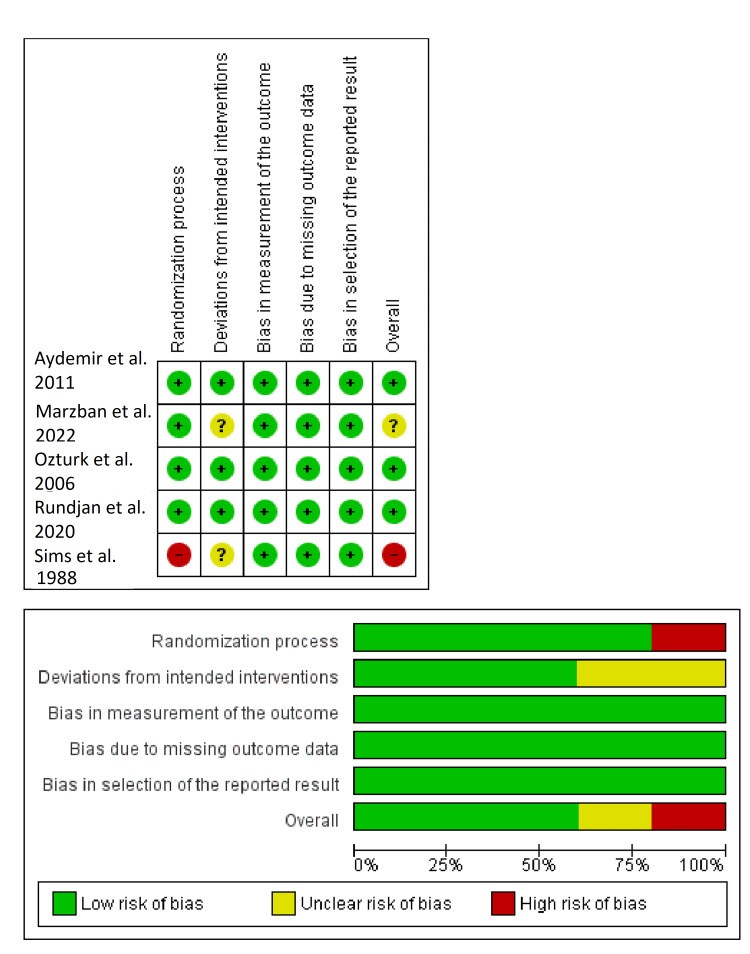
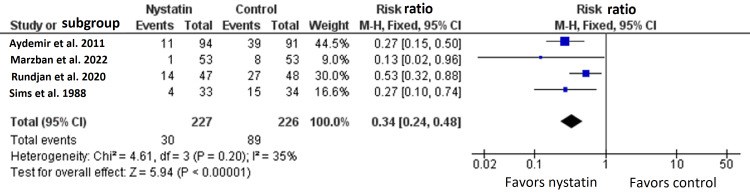
We conducted this systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the prophylactic role of oral nystatin in the prevention of fungal colonization in very low birth weight (VLBW) infants compared with placebo or no treatment intervention. From inception until June 2022, we screened four major databases for pertinent RCTs and examined their risk of bias. The main outcomes were the rate of fungal colonization, rate of invasive fungal infection, rate of mortality, mean length of stay in the neonatal intensive care unit (NICU), and mean duration of antibiotic treatment. We summarized data as risk ratio (RR) or mean difference (MD) with 95% confidence interval (CI), using the fixed-effects model. Five RCTs met our inclusion criteria. One RCT was evaluated as having "high risk," one RCT was evaluated as having "some concerns," and three RCTs were evaluated as having "low risk" of bias. Compared with the control group, oral nystatin prophylaxis was correlated with substantial decrease in the frequency of fungal colonization (n=4 RCTs, RR=0.34, 95% CI {0.24, 0.48}, p<0.0001), the rate of invasive fungal infection (n=4 RCTs, RR=0.15, 95% CI {0.12, 0.19}, p<0.0001), and the mean duration of antibiotic treatment (n=3 RCTs, MD=-2.79 days, 95% CI {-5.01, -0.56}, p=0.01). However, there was no significant difference between both groups regarding the rate of mortality (n=4 RCTs, RR=0.87, 95% CI {0.64, 1.18}, p=0.37) and mean length of stay in NICU (n=3 RCTs, MD=-2.85 days, 95% CI {-6.52, 0.82}, p=0.13). In conclusion, among VLBW infants, the prophylactic use of oral nystatin was correlated with favorable antifungal benefits compared with placebo or no treatment intervention.
Keywords: fungal colonization; fungal infection; low birth weight; meta-analysis; nystatin.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
Austin N, Darlow BA, McGuire W.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD003478. doi: 10.1002/14651858.CD003478.pub3.PMID: 19821309 Updated. Review.
Prophylactic oral antifungal agents to prevent systemic candida infection in preterm infants.
Austin NC, Darlow B.Cochrane Database Syst Rev. 2004;(1):CD003478. doi: 10.1002/14651858.CD003478.pub2.PMID: 14974017 Updated. Review.
Rundjan L, Wahyuningsih R, Oeswadi CA, Marsogi M, Purnamasari A.BMC Pediatr. 2020 Apr 17;20(1):170. doi: 10.1186/s12887-020-02074-0.PMID: 32303210 Free PMC article. Clinical Trial.
Austin N, Cleminson J, Darlow BA, McGuire W.Cochrane Database Syst Rev. 2015 Oct 24;2015(10):CD003478. doi: 10.1002/14651858.CD003478.pub5.PMID: 26497202 Free PMC article. Review.
Austin N, Darlow BA, McGuire W.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD003478. doi: 10.1002/14651858.CD003478.pub4.PMID: 23543519 Updated. Review.
KMEL References
References
-
- Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Blyth CC, Chen SC, Slavin MA, et al. Pediatrics. 2009;123:1360–1368. - PubMed
-
- Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Stoll BJ, Hansen N, Fanaroff AA, et al. Pediatrics. 2002;110:285–291. - PubMed
-
- Epidemiological features of nosocomial candidaemia in neonates, infants and children: a multicentre study in Iran. Ahangarkani F, Shokohi T, Rezai MS, et al. Mycoses. 2020;63:382–394. - PubMed
-
- Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Clin Infect Dis. 2004;39:309–317. - PubMed
-
- Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. JAMA. 2004;292:2357–2365. - PubMed
-
- Oral nystatin prophylaxis to prevent invasive candidiasis in neonatal intensive care unit. Ozturk MA, Gunes T, Koklu E, Cetin N, Koc N. Mycoses. 2006;49:484–492. - PubMed
-
- London, England: Cochrane; 2022. Cochrane Handbook for Systematic Reviews of Interventions.
-
- RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366 - PubMed
-
- Prophylactic oral nystatin and fungal infections in very-low-birthweight infants. Sims ME, Yoo Y, You H, Salminen C, Walther FJ. Am J Perinatol. 1988;5:33–36. - PubMed
-
- Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Aydemir C, Oguz SS, Dizdar EA, et al. Arch Dis Child Fetal Neonatal Ed. 2011;96:164–168. - PubMed
-
- Effectiveness of oral nystatin prophylaxis in the prevention of Candida colonization in very low birth weight preterm neonates; a randomized controlled trial. Marzban A, Aghdam MK, Ahadi S. https://ijn.mums.ac.ir/article_20231_8b10979d1f51d336501b4ec22608389c.pdf Iran J Neonatol. 2022;13:91–97.
-
- Clinical use of oral nystatin in the prevention of systemic candidosis in patients at particular risk. Schäfer-Korting M, Blechschmidt J, Korting HC. Mycoses. 1996;39:329–339. - PubMed
-
- Challenging issues in neonatal candidiasis. Kaufman DA. Curr Med Res Opin. 2010;26:1769–1778. - PubMed
-
- Prophylactic oral nystatin for preterm babies under 33 weeks' gestation decreases fungal colonisation and invasive fungaemia. Ganesan K, Harigopal S, Neal T, Yoxall CW. Arch Dis Child Fetal Neonatal Ed. 2009;94:275–278. - PubMed
-
- Oral nystatin prophylaxis and neonatal fungal infections. Howell A, Isaacs D, Halliday R. Arch Dis Child Fetal Neonatal Ed. 2009;94:429–433. - PubMed
-
- Chemoprophylaxis of neonatal fungal infections in very low birthweight infants: efficacy and safety of fluconazole and nystatin. Blyth CC, Barzi F, Hale K, Isaacs D. J Paediatr Child Health. 2012;48:846–851. - PubMed
-
- The global burden of fungal diseases. Vallabhaneni S, Mody RK, Walker T, Chiller T. Infect Dis Clin North Am. 2016;30:1–11. - PubMed