Hematopoietic Stem Cell Transplantation as Treatment for Patients with DOCK8 Deficiency
Susanne E Aydin 1, Alexandra F Freeman 2, Waleed Al-Herz 3, Hamoud A Al-Mousa 4, Rand K Arnaout 5, Roland C Aydin 1, Vincent Barlogis 6, Bernd H Belohradsky 7, Carmem Bonfim 8, Robbert G Bredius 9, Julia I Chu 10, Oana C Ciocarlie 11, Figen Doğu 12, Hubert B Gaspar 13, Raif S Geha 14, Andrew R Gennery 15, Fabian Hauck 1, Abbas Hawwari 16, Dennis D Hickstein 17, Manfred Hoenig 18, Aydan Ikinciogullari 12, Christoph Klein 1, Ashish Kumar 19, Marianne R S Ifversen 20, Susanne Matthes 21, Ayse Metin 22, Benedicte Neven 23, Sung-Yun Pai 24, Suhag H Parikh 25, Capucine Picard 26, Ellen D Renner 27, Özden Sanal 28, Ansgar S Schulz 18, Friedhelm Schuster 29, Nirali N Shah 30, Evan B Shereck 31, Mary A Slatter 32, Helen C Su 33, Joris van Montfrans 34, Wilhelm Woessmann 35, John B Ziegler 36, Michael H Albert 37; Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and the European Society for Primary Immunodeficiencies
Affiliations
Affiliations
- Dr von Hauner University Children's Hospital, Ludwig Maximilians Universität, Munich, Germany.
- National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md.
- Department of Pediatrics, Al-Sabah Hospital, Kuwait, Kuwait.
- Department of Pediatrics, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia.
- Department of Medicine, Allergy & Immunology, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia.
- Pediatric Hematology, Assistance publique des Hopitaux de Marseille, Marseille, France.
- Deutsche Selbsthilfe Angeborene Immundefekte, Schnaitsee, Germany.
- Pediatric Blood and Marrow Transplantation Program, Hospital de Clinicas, Federal University of Parana, Curitiba, Brazil.
- Pediatric Immunology, LUMC, Leiden, The Netherlands.
- Department of Pediatrics, Stanford University School of Medicine, Stanford, Calif.
- Department of Bone Marrow Transplantation, Great Ormond Street Hospital NHS Trust, London, United Kingdom.
- Department of Pediatric Immunology & Allergy, Ankara University School of Medicine, Ankara, Turkey.
- Molecular Immunology Unit, UCL Great Ormond Street Institute of Child Health, London, United Kingdom.
- Department of Immunology, Boston Children's Hospital, Boston, Mass.
- Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom.
- King Abdullah International Medical Research Center, Riyadh, Saudi Arabia.
- ETI/CCR/NCI, National Institutes of Health, Bethesda, Md.
- Department of Pediatrics, University Medical Center Ulm, Ulm, Germany.
- BMT/Immune Deficiency, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio.
- Department for Children and Adolescents, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
- Stem Cell Transplantation, St Anna Children's Hospital, Vienna, Austria.
- Pediatric Immunology, Ankara Children's Hematology Oncology Training and Research Hospital, Ankara, Turkey.
- Department for Pediatric Immuno-Hematology and Rheumatology, Necker Hospital, Paris, France.
- Boston Children's Hospital, Dana-Farber Cancer Institute, Boston, Mass.
- Pediatric Blood and Marrow Transplant Program, Duke University Medical Center, Durham, NC.
- Study Center of Primary Immunodeficiency, Necker Children's Hospital, Paris, France.
- Environmental Medicine, TU Munich, Neuherberg, Germany.
- Department of Pediatrics, Hacettepe University, Ankara, Turkey.
- Department of Pediatrics, Düsseldorf University Hospital, Düsseldorf, Germany.
- Pediatric Oncology Branch, National Cancer Institute, Bethesda, Md.
- Pediatric Hematology/Oncology, Oregon & Health Science University, Portland, Ore.
- Paediatric BMT, Great North Children's Hospital, Newcastle upon Tyne, United Kingdom.
- Laboratory of Clinical Immunology and Microbiology, NIAID, National Institutes of Health, Bethesda, Md.
- Pediatric Immunology and Infectious Diseases, UMC Utrecht, Utrecht, The Netherlands.
- Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
- Immunology & Infectious Diseases, Sydney Children's Hospital, Randwick, NSW, Australia.
- Dr von Hauner University Children's Hospital, Ludwig Maximilians Universität, Munich, Germany. Electronic address: malbert@med.lmu.de.
Abstract
Background: Biallelic variations in the dedicator of cytokinesis 8 (DOCK8) gene cause a combined immunodeficiency with eczema, recurrent bacterial and viral infections, and malignancy. Natural disease outcome is dismal, but allogeneic hematopoietic stem cell transplantation (HSCT) can cure the disease.
Objective: To determine outcome of HSCT for DOCK8 deficiency and define possible outcome variables.
Methods: We performed a retrospective study of the results of HSCT in a large international cohort of DOCK8-deficient patients.
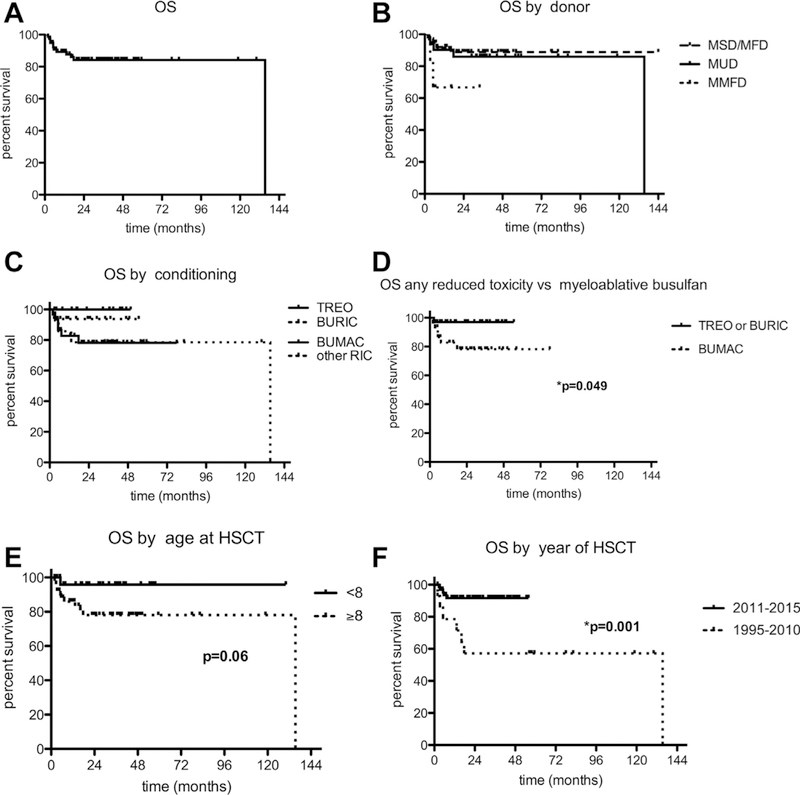
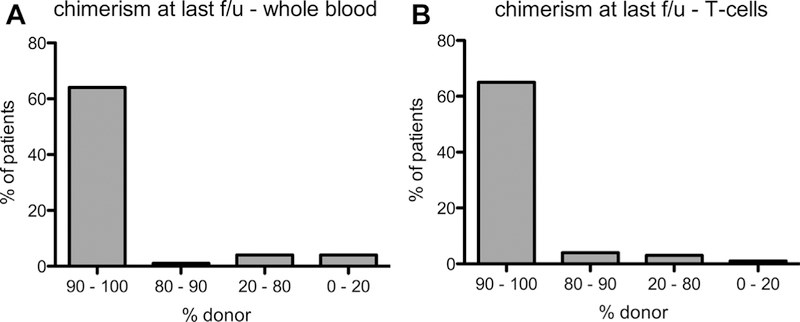
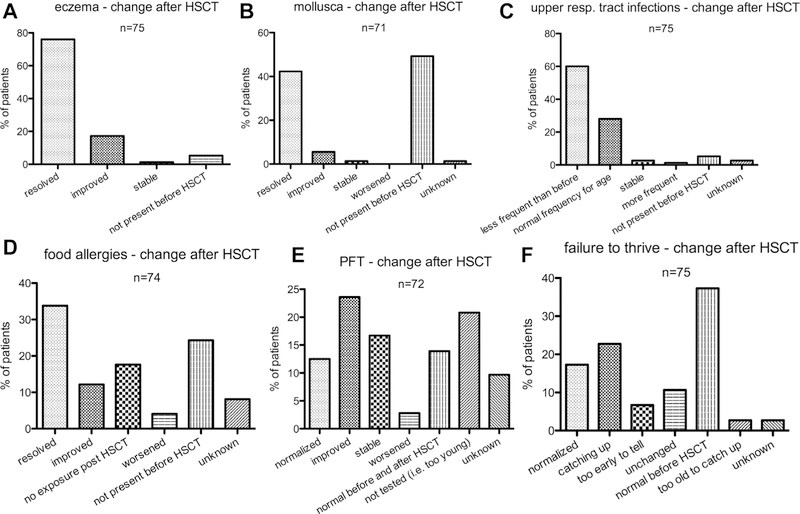
Results: We identified 81 patients from 22 centers transplanted at a median age of 9.7 years (range, 0.7-27.2 years) between 1995 and 2015. After median follow-up of 26 months (range, 3-135 months), 68 (84%) patients are alive. Severe acute (III-IV) or chronic graft versus host disease occurred in 11% and 10%, respectively. Causes of death were infections (n = 5), graft versus host disease (5), multiorgan failure (2), and preexistent lymphoma (1). Survival after matched related (n = 40) or unrelated (35) HSCT was 89% and 81%, respectively. Reduced-toxicity conditioning based on either treosulfan or reduced-dose busulfan resulted in superior survival compared with fully myeloablative busulfan-based regimens (97% vs 78%; P = .049). Ninety-six percent of patients younger than 8 years at HSCT survived, compared with 78% of those 8 years and older (P = .06). Of the 73 patients with chimerism data available, 65 (89%) had more than 90% donor T-cell chimerism at last follow-up. Not all disease manifestations responded equally well to HSCT: eczema, infections, and mollusca resolved quicker than food allergies or failure to thrive.
Conclusions: HSCT is curative in most DOCK8-deficient patients, confirming this approach as the treatment of choice. HSCT using a reduced-toxicity regimen may offer the best chance for survival.
Keywords: Combined immunodeficiency; DOCK8 deficiency; HSCT.
Conflict of interest statement
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
Figures
Similar articles
Shah NN, Freeman AF, Su H, Cole K, Parta M, Moutsopoulos NM, Baris S, Karakoc-Aydiner E, Hughes TE, Kong HH, Holland SM, Hickstein DD.Biol Blood Marrow Transplant. 2017 Jun;23(6):980-990. doi: 10.1016/j.bbmt.2017.03.016. Epub 2017 Mar 10.PMID: 28288951 Free PMC article.
Al-Herz W, Chu JI, van der Spek J, Raghupathy R, Massaad MJ, Keles S, Biggs CM, Cockerton L, Chou J, Dbaibo G, Elisofon SA, Hanna-Wakim R, Kim HB, Lehmann LE, McDonald DR, Notarangelo LD, Veys P, Chatila TA, Geha RS, Gaspar HB, Pai SY.J Allergy Clin Immunol. 2016 Sep;138(3):852-859.e3. doi: 10.1016/j.jaci.2016.02.022. Epub 2016 Apr 6.PMID: 27130861 Free PMC article.
Raedler J, Magg T, Rohlfs M, Klein C, Vallée T, Hauck F, Albert MH.J Clin Immunol. 2021 Oct;41(7):1536-1548. doi: 10.1007/s10875-021-01069-5. Epub 2021 Jun 2.PMID: 34080085 Free PMC article.
Petersen SL.Dan Med Bull. 2007 May;54(2):112-39.PMID: 17521527 Review.
DOCK8 deficiency: Insights into pathophysiology, clinical features and management.
Biggs CM, Keles S, Chatila TA.Clin Immunol. 2017 Aug;181:75-82. doi: 10.1016/j.clim.2017.06.003. Epub 2017 Jun 15.PMID: 28625885 Free PMC article. Review.
Cited by
Kono A, Wakamatsu M, Umezawa Y, Muramatsu H, Fujiwara H, Tomomasa D, Inoue K, Hattori K, Mitsui T, Takada H, Minegishi Y, Takahashi Y, Yamamoto M, Mori T, Kanegane H.Int J Hematol. 2023 May 3. doi: 10.1007/s12185-023-03613-y. Online ahead of print.PMID: 37131080
Personalized hematopoietic stem cell transplantation for inborn errors of immunity.
Slatter M, Lum SH.Front Immunol. 2023 Apr 5;14:1162605. doi: 10.3389/fimmu.2023.1162605. eCollection 2023.PMID: 37090739 Free PMC article. Review.
Giancotta C, Colantoni N, Pacillo L, Santilli V, Amodio D, Manno EC, Cotugno N, Rotulo GA, Rivalta B, Finocchi A, Cancrini C, Diociaiuti A, El Hachem M, Zangari P.Front Pediatr. 2023 Mar 22;11:1129249. doi: 10.3389/fped.2023.1129249. eCollection 2023.PMID: 37033173 Free PMC article. Review.
Diagnostic challenge in a series of eleven patients with hyper IgE syndromes.
Yaakoubi R, Mekki N, Ben-Mustapha I, Ben-Khemis L, Bouaziz A, Ben Fraj I, Ammar J, Hamzaoui A, Turki H, Boussofara L, Denguezli M, Haddad S, Ouederni M, Bejaoui M, Chan KW, Lau YL, Mellouli F, Barbouche MR, Ben-Ali M.Front Immunol. 2023 Jan 10;13:1057679. doi: 10.3389/fimmu.2022.1057679. eCollection 2022.PMID: 36703986 Free PMC article.
Zhang L, Cao Y, Dai X, Zhang X.Front Pharmacol. 2022 Nov 1;13:1065029. doi: 10.3389/fphar.2022.1065029. eCollection 2022.PMID: 36386145 Free PMC article. Review.