Cutaneous barrier leakage and gut inflammation drive skin disease in Omenn syndrome
Rosita Rigoni 1, Elena Fontana 2, Kerry Dobbs 3, Veronica Marrella 1, Valentina Taverniti 4, Virginia Maina 1, Amanda Facoetti 5, Giovanna D'Amico 6, Waleed Al-Herz 7, Mario Ernesto Cruz-Munoz 8, Catharina Schuetz 9, Andrew R Gennery 10, Elizabeth K Garabedian 11, Silvia Giliani 12, Deborah Draper 3, Ghassan Dbaibo 13, Raif S Geha 14, Isabelle Meyts 15, Thomas Tousseyn 16, Benedicte Neven 17, Despina Moshous 17, Alain Fischer 17, Ansgar Schulz 18, Andrea Finocchi 19, Douglas B Kuhns 20, Danielle L Fink 20, Michail S Lionakis 21, Muthulekha Swamydas 21, Simone Guglielmetti 4, Julie Alejo 22, Ian A Myles 3, Stefania Pittaluga 22, Luigi D Notarangelo 23, Anna Villa 24, Barbara Cassani 25
Affiliations
Affiliations
- Milan Unit, Institute for Genetic and Biomedical Research (IRGB) National Research Council (CNR), Milan, Italy; Humanitas Clinical and Research Center IRCCS, Rozzano, Milan, Italy.
- Humanitas Clinical and Research Center IRCCS, Rozzano, Milan, Italy.
- Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Bethesda, Md.
- Department of Food, Environmental, and Nutritional Sciences, University of Milan Milan, Italy.
- Humanitas Clinical and Research Center IRCCS, Rozzano, Milan, Italy; Humanitas University, Rozzano, Milan, Italy.
- Centro Ricerca Tettamanti, Clinica Pediatrica, Università Milano-Bicocca, Monza, Italy.
- Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait; Allergy and Clinical Immunology Unit, Pediatric Department, Al-Sabah Hospital, Kuwait City, Kuwait.
- Facultad de Medicina, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico.
- Department of Pediatrics, Medizinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany.
- Great North Children's Hospital, Clinical Resource Building, Newcastle upon Tyne, United Kingdom; Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom.
- National Human Genome Research Institute, NIH, Bethesda, Md.
- Department of Molecular and Translational Medicine, University of Brescia, Brescia, Italy; Cytogenetic and Medical Genetics Unit, "A. Nocivelli" Institute for Molecular Medicine, Spedali Civili Hospital, Brescia, Italy.
- Department of Pediatrics and Adolescent Medicine, American University of Beirut Medical Center, Beirut, Lebanon.
- Division of Immunology, Boston Children's Hospital, Harvard Medical School, Boston, Mass.
- Department of Pediatrics, Universitair Ziekenhuis Leuven, University Hospitals Leuven, Leuven, Belgium; Laboratory for Inborn Errors of Immunity, Department of Immunology, Microbiology and Transplantation, Katholieke Universiteit Leuven, Leuven, Belgium.
- Lab for Translational Cell and Tissue Research, Department of Imaging and Pathology, Katholieke Universiteit Leuven, Leuven, Belgium.
- Imagine Institute, Paris Descartes-Sorbonne Paris Cité University, Paris, France; Pediatric Immuno-Hematology Unit, Necker Children Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France.
- Department of Pediatrics and Adolescent Medicine, University Medical Center Ulm, Ulm, Germany.
- Department of Pediatrics, Children's Hospital Bambino Gesù, Rome, Italy.
- Neutrophil Monitoring Laboratory, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, Md.
- Fungal Pathogenesis Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
- Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Md.
- Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Bethesda, Md. Electronic address: luigi.notarangelo2@nih.gov.
- Milan Unit, Institute for Genetic and Biomedical Research (IRGB) National Research Council (CNR), Milan, Italy; Telethon Institute for Gene Therapy, Division of Regenerative Medicine, Stem Cells, and Gene Therapy, IRCCS San Raffaele Scientific Institute, Milan, Italy. Electronic address: villa.anna@hsr.it.
- Milan Unit, Institute for Genetic and Biomedical Research (IRGB) National Research Council (CNR), Milan, Italy; Humanitas Clinical and Research Center IRCCS, Rozzano, Milan, Italy. Electronic address: barbara.cassani@humanitasresearch.it.
Abstract
Background: Severe early-onset erythroderma and gut inflammation, with massive tissue infiltration of oligoclonal activated T cells are the hallmark of Omenn syndrome (OS).
Objective: The impact of altered gut homeostasis in the cutaneous manifestations of OS remains to be clarified.
Methods: We analyzed a cohort of 15 patients with OS and the 129Sv/C57BL/6 knock-in Rag2R229Q/R229Q (Rag2R229Q) mouse model. Homing phenotypes of circulating lymphocytes were analyzed by flow cytometry. Inflammatory cytokines and chemokines were examined in the sera by ELISA and in skin biopsies by immunohistochemistry and in situ RNA hybridization. Experimental colitis was induced in mice by dextran sulfate sodium salt.
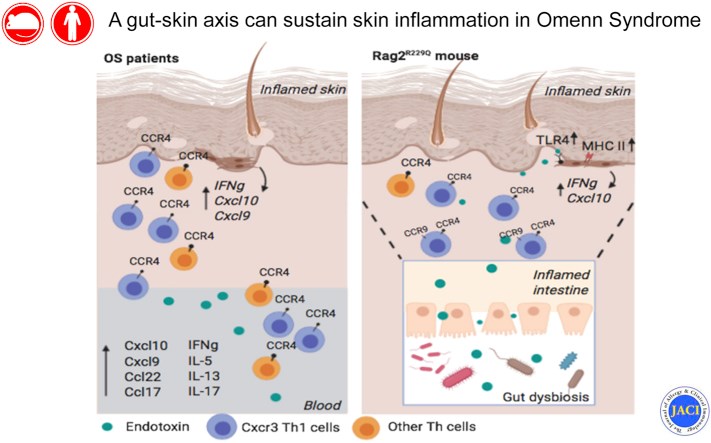
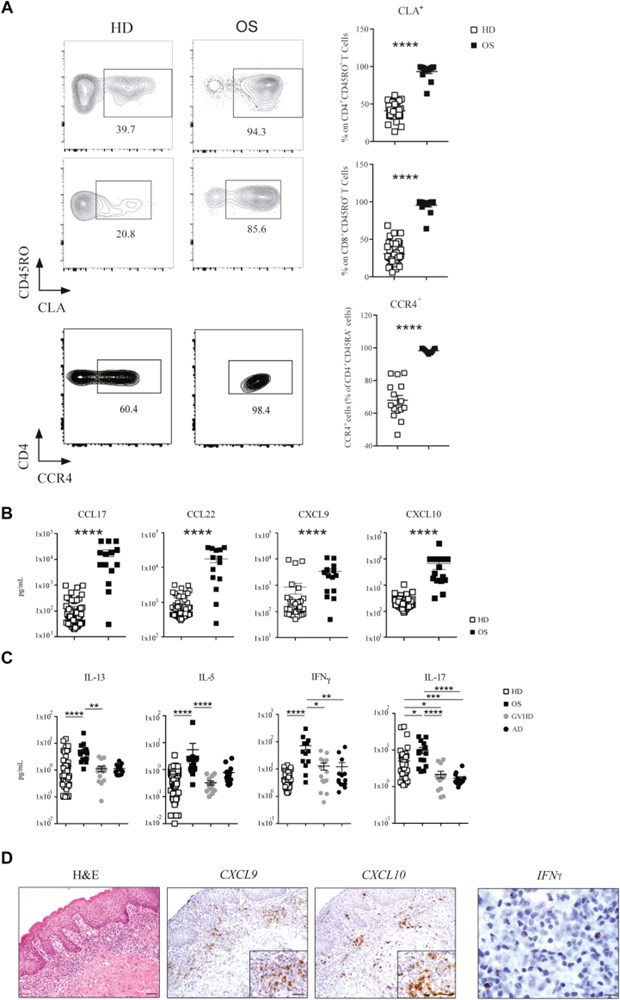
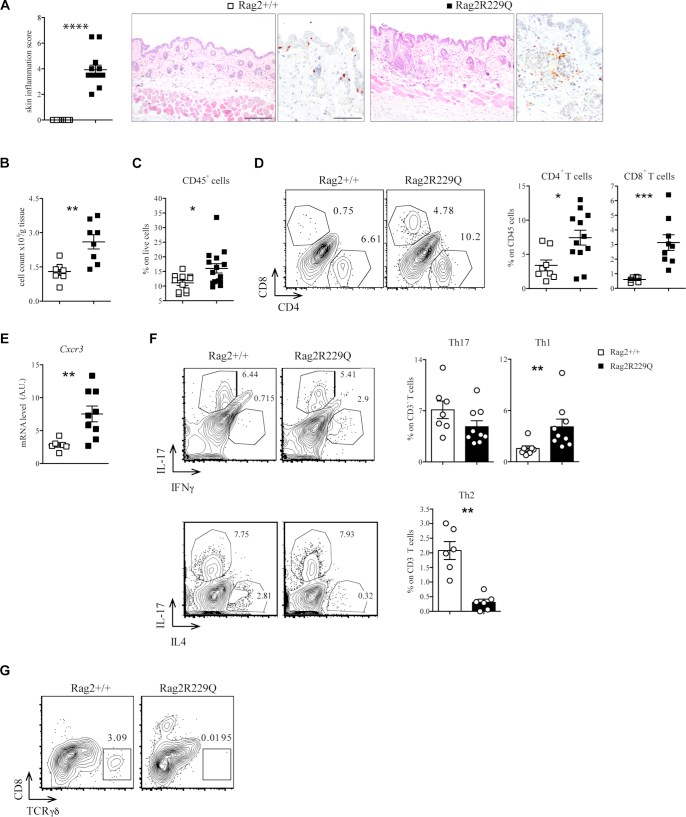
Results: We show that memory/activated T cells from patients with OS and from the Rag2R229Q mouse model of OS abundantly express the skin homing receptors cutaneous lymphocyte associated antigen and CCR4 (Ccr4), associated with high levels of chemokine C-C motif ligands 17 and 22. Serum levels of LPS are also elevated. A broad Th1/Th2/Th17 inflammatory signature is detected in the periphery and in the skin. Increased Tlr4 expression in the skin of Rag2R229Q mice is associated with enhanced cutaneous inflammation on local and systemic administration of LPS. Likewise, boosting colitis in Rag2R229Q mice results in increased frequency of Ccr4+ splenic T cells and worsening of skin inflammation, as indicated by epidermal thickening, enhanced epithelial cell activation, and dermal infiltration by Th1 effector T cells.
Conclusions: These results support the existence of an interplay between gut and skin that can sustain skin inflammation in OS.
Keywords: LPS; RAG; T cells; chemokines; cytokines; dysbiosis; erythroderma; gut-skin axis; immune-mediated disease; skin inflammation.
Figures
Similar articles
Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects.
Rigoni R, Fontana E, Guglielmetti S, Fosso B, D'Erchia AM, Maina V, Taverniti V, Castiello MC, Mantero S, Pacchiana G, Musio S, Pedotti R, Selmi C, Mora JR, Pesole G, Vezzoni P, Poliani PL, Grassi F, Villa A, Cassani B.J Exp Med. 2016 Mar 7;213(3):355-75. doi: 10.1084/jem.20151116. Epub 2016 Feb 29.PMID: 26926994 Free PMC article.
Hypomorphic mutation in the RAG2 gene affects dendritic cell distribution and migration.
Maina V, Marrella V, Mantero S, Cassani B, Fontana E, Anselmo A, Del Prete A, Sozzani S, Vezzoni P, Poliani PL, Villa A.J Leukoc Biol. 2013 Dec;94(6):1221-30. doi: 10.1189/jlb.0713365. Epub 2013 Sep 19.PMID: 24052573
Capo V, Castiello MC, Fontana E, Penna S, Bosticardo M, Draghici E, Poliani LP, Sergi Sergi L, Rigoni R, Cassani B, Zanussi M, Carrera P, Uva P, Dobbs K, Sacchetti N, Notarangelo LD, van Til NP, Wagemaker G, Villa A.J Allergy Clin Immunol. 2018 Sep;142(3):928-941.e8. doi: 10.1016/j.jaci.2017.11.015. Epub 2017 Dec 11.PMID: 29241731 Free PMC article.
Omenn syndrome does not live by V(D)J recombination alone.
Marrella V, Maina V, Villa A.Curr Opin Allergy Clin Immunol. 2011 Dec;11(6):525-31. doi: 10.1097/ACI.0b013e32834c311a.PMID: 22001740 Review.
[Omenn Syndrome and DNA recombination defects].
Yachie A.Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(3):179-189. doi: 10.2177/jsci.40.179.PMID: 28747605 Review. Japanese.
Cited by
Intestinal inflammation alters the antigen-specific immune response to a skin commensal.
Merana GR, Dwyer LR, Dhariwala MO, Weckel A, Gonzalez JR, Okoro JN, Cohen JN, Tamaki CM, Han J, Tasoff P, Palacios-Calderon Y, Ha CWY, Lynch SV, Segre JA, Kong HH, Kattah MG, Ma A, Scharschmidt TC.Cell Rep. 2022 May 31;39(9):110891. doi: 10.1016/j.celrep.2022.110891.PMID: 35649365 Free PMC article.
Fang Z, Li L, Zhang H, Zhao J, Lu W, Chen W.Front Immunol. 2021 Jul 14;12:720393. doi: 10.3389/fimmu.2021.720393. eCollection 2021.PMID: 34335634 Free PMC article. Review.
RAG deficiencies: Recent advances in disease pathogenesis and novel therapeutic approaches.
Bosticardo M, Pala F, Notarangelo LD.Eur J Immunol. 2021 May;51(5):1028-1038. doi: 10.1002/eji.202048880. Epub 2021 Mar 22.PMID: 33682138 Free PMC article. Review.
Innovative Cell-Based Therapies and Conditioning to Cure RAG Deficiency.
Villa A, Capo V, Castiello MC.Front Immunol. 2020 Nov 19;11:607926. doi: 10.3389/fimmu.2020.607926. eCollection 2020.PMID: 33329604 Free PMC article. Review.
KMEL References
References
-
- Gebhardt T., Whitney P.G., Zaid A., Mackay L.K., Brooks A.G., Heath W.R. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. - PubMed
-
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. - PubMed
-
- Homey B., Alenius H., Muller A., Soto H., Bowman E.P., Yuan W. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. - PubMed
-
- Mora J.R., Von Andrian U.H. Specificity and plasticity of memory lymphocyte migration. Curr Top Microbiol Immunol. 2006;308:83–116. - PubMed
-
- Hart A.L., Ng S.C., Mann E., Al-Hassi H.O., Bernardo D., Knight S.C. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm Bowel Dis. 2010;16:1969–1977. - PubMed
-
- Lehman H. Skin manifestations of primary immune deficiency. Clin Rev Allergy Immunol. 2014;46:112–119. - PubMed
-
- Scheimberg I., Hoeger P.H., Harper J.I., Lake B., Malone M. Omenn's syndrome: differential diagnosis in infants with erythroderma and immunodeficiency. Pediatr Dev Pathol. 2001;4:237–245. - PubMed
-
- Maina V., Marrella V., Mantero S., Cassani B., Fontana E., Anselmo A. Hypomorphic mutation in the RAG2 gene affects dendritic cell distribution and migration. J Leukoc Biol. 2013;94:1221–1230. - PubMed
-
- Smith P.D., MacDonald T.T., Blumberg R.S., Society for Mucosal Immunology . Garland Science/Taylor & Francis Group; London: 2013. Principles of mucosal immunology.
-
- Parodi A., Paolino S., Greco A., Drago F., Mansi C., Rebora A. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759–764. - PubMed
-
- Wang M., Karlsson C., Olsson C., Adlerberth I., Wold A.E., Strachan D.P. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121:129–134. - PubMed
-
- Fuhlbrigge R.C., Kieffer J.D., Armerding D., Kupper T.S. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. - PubMed
-
- Biedermann T., Schwarzler C., Lametschwandtner G., Thoma G., Carballido-Perrig N., Kund J. Targeting CLA/E-selectin interactions prevents CCR4-mediated recruitment of human Th2 memory cells to human skin in vivo. Eur J Immunol. 2002;32:3171–3180. - PubMed
-
- Schwarz A., Bruhs A., Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017;137:855–864. - PubMed
-
- Komine M., Rao L.S., Freedberg I.M., Simon M., Milisavljevic V., Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J Invest Dermatol. 2001;116:330–338. - PubMed
-
- Pasparakis M., Haase I., Nestle F.O. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. - PubMed
-
- Elias P.M. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. - PubMed
-
- Liao S., Ruddle N.H. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. - PubMed
-
- Albanesi C., Scarponi C., Sebastiani S., Cavani A., Federici M., Sozzani S. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J Leukoc Biol. 2001;70:617–623. - PubMed
-
- Fierro M.T., Comessatti A., Quaglino P., Ortoncelli M., Osella Abate S., Ponti R. Expression pattern of chemokine receptors and chemokine release in inflammatory erythroderma and Sezary syndrome. Dermatology. 2006;213:284–292. - PubMed
-
- Lonsdorf A.S., Hwang S.T., Enk A.H. Chemokine receptors in T-cell-mediated diseases of the skin. J Invest Dermatol. 2009;129:2552–2566. - PubMed
-
- Di Cesare A., Di Meglio P., Nestle F.O. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–2571. - PubMed
-
- Lim H.W., Lee J., Hillsamer P., Kim C.H. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. - PubMed
-
- Andrew D.P., Ruffing N., Kim C.H., Miao W., Heath H., Li Y. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. - PubMed
-
- Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. New Engl J Med. 2006;354:610–621. - PubMed
-
- Subramaniam J.M., Whiteside G., McKeage K., Croxtall J.C. Mogamulizumab: first global approval. Drugs. 2012;72:1293–1298. - PubMed
-
- Song P.I., Park Y.M., Abraham T., Harten B., Zivony A., Neparidze N. Human keratinocytes express functional CD14 and Toll-like receptor 4. J Invest Dermatol. 2002;119:424–432. - PubMed
-
- Leffler D.A., Green P.H., Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561–571. - PubMed
-
- Weinstock L.B., Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875–876. - PubMed
-
- Orange J.S. Congenital immunodeficiencies and sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S99–S107. - PubMed