Management of anaphylaxis due to COVID-19 vaccines in the elderly
Jean Bousquet 1 2 3, Ioana Agache 4, Hubert Blain 5, Marek Jutel 6 7, Maria Teresa Ventura 8, Margitta Worm 1, Stefano Del Giacco 9, Athanasios Benetos 10, Beatrice Maria Bilo 11 12, Wienczyslawa Czarlewski 13, Amir Hamzah Abdul Latiff 14, Mona Al-Ahmad 15 16, Elizabeth Angier 17, Isabella Annesi-Maesano 18, Marina Atanaskovic-Markovic 19, Claus Bachert 20 21 22 23, Annick Barbaud 24 25, Anna Bedbrook 14, Kazi S Bennoor 26, Elena Camelia Berghea 27 28, Carsten Bindslev-Jensen 29, Sergio Bonini 30, Sinthia Bosnic-Anticevich 31 32, Knut Brockow 33, Luisa Brussino 34, Paulo Camargos 35, G Walter Canonica 36 37, Victoria Cardona 38 39, Pedro Carreiro-Martins 40 41, Ana Carriazo 42, Thomas Casale 43, Jean-Christoph Caubet 44, Lorenzo Cecchi 45, Antonio Cherubini 46, George Christoff 47, Derek K Chu 48, Alvaro A Cruz 49, Dejan Dokic 50, Yehia El-Gamal 51, Motohiro Ebisawa 52, Bernadette Eberlein 33, John Farrell 53, Montserrat Fernandez-Rivas 54, Wytske J Fokkens 55 56, Joao A Fonseca 57 58, Yadong Gao 59, Gaëtan Gavazzi 60, Radoslaw Gawlik 61, Asli Gelincik 62, Bilun Gemicioğlu 63, Maia Gotua 64, Olivier Guérin 65, Tari Haahtela 66, Karin Hoffmann-Sommergruber 67, Hans Jürgen Hoffmann 68 69, Maja Hofmann 1, Martin Hrubisko 70, Maddalena Illario 71, Carla Irani 72, Zhanat Ispayeva 73, Juan Carlos Ivancevich 74, Kaja Julge 75, Igor Kaidashev 76, Musa Khaitov 77, Edward Knol 78, Helga Kraxner 79, Piotr Kuna 80, Violeta Kvedariene 81 82, Antti Lauerma 83, Lan T T Le 84, Vincent Le Moing 85, Michael Levin 86, Renaud Louis 87, Olga Lourenco 88, Vera Mahler 89, Finbarr C Martin 90, Andrea Matucci 91, Branislava Milenkovic 92, Stéphanie Miot 5, Emma Montella 71, Mario Morais-Almeida 93, Charlotte G Mortz 29, Joaquim Mullol 94 95, Leyla Namazova-Baranova 96, Hugo Neffen 97, Kristof Nekam 98, Marek Niedoszytko 99, Mikaëla Odemyr 100, Robyn E O'Hehir 101, Yoshitaka Okamoto 102, Markus Ollert 103 104, Oscar Palomares 105, Nikolaos G Papadopoulos 106, Petr Panzner 107, Giovanni Passalacqua 108, Vincenzo Patella 109, Mirko Petrovic 110, Oliver Pfaar 111, Nhân Pham-Thi 112, Davor Plavec 113 114, Todor A Popov 115, Marysia T Recto 116, Frederico S Regateiro 117 118 119, Jacques Reynes 85, Regina E Roller-Winsberger 120, Yves Rolland 121, Antonino Romano 122 123, Carmen Rondon 124 125, Menachem Rottem 126 127, Philip W Rouadi 128, Nathalie Salles 129, Boleslaw Samolinski 130, Alexandra F Santos 131 132 133 134, Faradiba S Sarquis 135, Joaquin Sastre 136, Jos M G A Schols 137, Nicola Scichilone 138, Anna Sediva 139, Mohamed H Shamji 140 141, Aziz Sheikh 142, Isabel Skypala 143, Sylwia Smolinska 144 145, Milena Sokolowska 146, Bernardo Sousa-Pinto 57 58 147, Milan Sova 148, Rafael Stelmach 149, Gunter Sturm 150 151, Charlotte Suppli Ulrik 152, Ana Maria Todo-Bom 153, Sanna Toppila-Salmi 66, Ioanna Tsiligianni 154 155, Maria Torres 156, Eva Untersmayr 67, Marilyn Urrutia Pereira 157, Arunas Valiulis 158 159, Joana Vitte 160 161, Alessandra Vultaggio 91, Dana Wallace 162, Jolanta Walusiak-Skorupa 163, De-Yun Wang 164, Susan Waserman 48, Arzu Yorgancioglu 165, Osman M Yusuf 166, Mario Zernotti 167, Mihaela Zidarn 168, Tomas Chivato 169, Cezmi A Akdis 170, Torsten Zuberbier 1, Ludger Klimek 171 172
Affiliations
Affiliations
- 1Department of Dermatology and Allergy, Comprehensive Allergy Center, Charité Universitätsmedizin Berlin, Humboldt-Universität zu Berlin, Berlin Institute of Health, Berlin, Germany.
- 2University Hospital Montpellier, France.
- 3MACVIA-France, Montpellier, France.
- 4Faculty of Medicine, Transylvania University, Brasov, Romania.
- 5Department of Geriatrics, Montpellier University Hospital, Montpellier, France.
- 6Department of Clinical Immunology, Wrocław Medical University, Wroclaw, Poland.
- 7ALL-MED Medical Research Institute, Wrocław, Poland.
- 8University of Bari Medical School, Unit of Geriatric Immunoallergology, Bari, Italy.
- 9Department of Medical Sciences and Public Health, Unit of Allergy and Clinical Immunology, University Hospital "Duilio Casula", University of Cagliari, Cagliari, Italy.
- 10Department of Geriatrics, CHRU de Nancy and Inserm DCAC, Université de Lorraine, Nancy, France.
- 11Allergy Unit, Department of Clinical and Molecular Sciences, Università Politecnica delle Marche, Ancona, Italy.
- 12Department of Internal Medicine, University Hospital, Ospedali Riuniti di Ancona, Ancona, Italy.
- 13Medical Consulting Czarlewski, Levallois, France.
- 14Department of Pediatrics, Allergy & Immunology Centre, Pantai Hospital, Universiti Putra Malaysia Teaching Hospital, Kuala Lumpur, Malaysia.
- 15Microbiology Department, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait.
- 16Department of Allergy, Al-Rashed Allergy Center, Kuwait City, Kuwait.
- 17Primary Care and Population Sciences, University of Southampton, Southampton, UK.
- 18Institut Desbrest d'Epidémiologie et Santé Publique (IDESP), INSERM et Université de Montpellier, Montpellier, France.
- 19Faculty of Medicine, University Children's Hospital, University of Belgrade, Belgrade, Serbia.
- 20Upper Airways Research Laboratory, ENT Department, Ghent University Hospital, Ghent, Belgium.
- 21International Airway Research Center, Sun Yat-sen University, First Affiliated Hospital Guangzou, China.
- 22Division of ENT Diseases, CLINTEC, Karolinska Institutet, Stockholm, Sweden.
- 23Department of ENT Diseases, Karolinska University Hospital, Stockholm, Sweden.
- 24Division of Service de Dermatologie et Allergologie, Hôpital Tenon, Paris, France.
- 25Division of Equipe PEPITES, Sorbonne Université, Institut Pierre Louis d'Epidémiologie et de Santé Publique, Paris, France.
- 26Department of Respiratory Medicine, National Institute of Diseases of the Chest and Hospital, Dhaka, Bangladesh.
- 27Allergology and Clinical Immunology, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
- 28Clinical Emergency Hospital for Children MS Curie, Bucharest, Romania.
- 29Department of Dermatology and Allergy Centre, Odense University Hospital, Odense Research Center for Anaphylaxis (ORCA), Odense, Denmark.
- 30Institute of Translational Pharmacology, Italian National Research Council, Rome, Italy.
- 31Woolcock Institute of Medical Research, University of Sydney, Glebe, NSW, Australia.
- 32Woolcock Emphysema Centre, Sydney Local Health District, Glebe, NSW, Australia.
- 33Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Munich, Germany.
- 34Department of Medical Sciences, Allergy and Clinical Immunology Unit, University of Torino & Mauriziano Hospital, Torino, Italy.
- 35Department of Pediatrics, Medical School, Federal University of Minas Gerais, Belo Horizonte, Brazil.
- 36Department of Biomedical Sciences, Humanitas University, Milan, Italy.
- 37IRCCS Humanitas Research Hospital, Personalized Medicine, Asthma and Allergy, Milan, Italy.
- 38Allergy Section, Department of Internal Medicine, Hospital Vall d'Hebron, Barcelona, Spain.
- 39ARADyAL Research Network, Barcelona, Spain.
- 40Serviço de Imunoalergologia, Hospital de Dona Estefânia, Centro Hospitalar de Lisboa Central, Lisbon, Portugal.
- 41Faculdade de Ciências Médicas (FCM), CEDOC, Universidade Nova de Lisboa, Lisbon, Portugal.
- 42Regional Ministry of Health of Andalusia, Seville, Spain.
- 43Division of Allergy/Immunology, University of South Florida, Tampa, FL, USA.
- 44Pediatric Allergy Unit, Department of Child and Adolescent, Geneva University Hospital, Geneva, Switzerland.
- 45SOS Allergology and Clinical Immunology, USL Toscana Centro, Prato, Italy.
- 46Geriatria, Accettazione Geriatrica e Centro di Ricerca per l'invecchiamento, IRCCS INRCA, Ancona, Italy.
- 47Faculty of Public Health, Medical University, Sofia, Bulgaria.
- 48Department of Medicine and Health Research Methods, Evidence & Impact, McMaster University, Hamilton, ON, Canada.
- 49Fundação ProAR, Federal University of Bahia and GARD/WHO Planning Group, Salvador, BA, Brazil.
- 50Medical Faculty, University Clinic of Pulmology and Allergy, Skopje, Republic of Macedonia.
- 51Pediatric Allergy and Immunology Unit, Children's Hospital, Ain Shams University, Cairo, Egypt.
- 52Clinical Research Center for Allergy and Rheumatology, NHO Sagamihara National Hospital, Sagamihara, Japan.
- 53LANUA International Healthcare Consultancy, Down, UK.
- 54Allergy Department Hospital Clínico San Carlos, UCM, IdISSC, Madrid, Spain.
- 55Department of Otorhinolaryngology, Academic Medical Centers, AMC, Amsterdam, The Netherland.
- 56EUFOREA, Brussels, Belgium.
- 57Faculdade de Medicina, CINTESIS, Center for Health Technology and Services Research, Universidade do Porto, Porto, Portugal.
- 58Allergy Unit, CUF Porto, Porto, Portugal.
- 59Department of Allergology, Zhongnan Hospital of Wuhan University, Wuhan, China.
- 60Service Gériatrie Clinique, Centre Hospitalo-Universitaire Grenoble-Alpes, GREPI (TIMC-IMAG, CNRS 5525, Université Grenoble-Alpes, Grenoble, France.
- 61Department of Internal Medicine, Allergy and Clin Immunology, Silesian University of Medicine, Katowice, Poland.
- 62Division of Immunology and Allergic Diseases, Department of Internal Medicine, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey.
- 63Department of Pulmonary Diseases, Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Istanbul, Turkey.
- 64Center of Allergy and Immunology, Georgian Association of Allergology and Clinical Immunology, Tbilisi, Georgia.
- 65Service de Gériatrie, CHRU, Nice, France.
- 66Skin and Allergy Hospital, Helsinki University Hospital, University of Helsinki University, Helsinki, Finland.
- 67Institute of Pathophysiology and Allergy Research, Center of Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Vienna, Austria.
- 68Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Aarhus, Denmark.
- 69Institute of Clinical Medicine, Aarhus University, Aarhus, Denmark.
- 70Department of Clinical Immunology and Allergy, Oncology Institute of St Elisabeth, Bratislava, Slovakia.
- 71Department of Public Health and Research and Development Unit, Federico II University & Hospital, Naples, Italy.
- 72Department of Internal Medicine and Infectious Diseases, St Joseph University, Hotel Dieu de France Hospital, Beirut, Lebanon.
- 73President of Kazakhstan Association of Allergology and Clinical Immunology, Department of Allergology and Clinical Immunology, Kazakh National Medical University, Almaty, Kazakhstan.
- 74Servicio de Alergia e Immunologia, Clinica Santa Isabel, Buenos Aires, Argentina.
- 75Tartu University Institute of Clinical Medicine, Children's Clinic, Tartu, Estonia.
- 76Ukrainina Medical Stomatological Academy, Poltava, Ukraine.
- 77National Research Center, Institute of Immunology, Federal Medicobiological Agency, Laboratory of Molecular Immunology, Moscow, Russia.
- 78Departments of Immunology and Dermatology/Allergology, University Medical Center, Utrecht, The Netherlands.
- 79Department of Otorhinolaryngology, Head and Neck Surgery, Semmelweis University, Budapest, Hungary.
- 80Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, Lodz, Poland.
- 81Institute of Biomedical Sciences, Department of Pathology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
- 82Institute of Clinical Medicine, Clinic of Chest Diseases and Allergology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
- 83Department of Dermatology and Allergology, University of Helsinki and Helsinki University, Helsinki, Finland.
- 84University of Medicine and Pharmacy, Hochiminh City, Vietnam.
- 85Department of Infectiology, Montpellier University Hospital, Montpellier, France.
- 86Division Paediatric Allergology, University of Cape Town, Cape Town, South Africa.
- 87Department of Pulmonary Medicine, CHU Sart-Tilman, GIGA I3 Research Group, Liege, Belgium.
- 88Faculty of Health Sciences, CICS - UBI, Health Sciences Research Centre, University of Beira Interior, Covilhã, Portugal.
- 89Paul-Ehrlich-Institut, Langen, Germany.
- 90Emeritus Geriatrician and Professor of Medical Gerontology Population Health Sciences I, King's College London, London, UK.
- 91Immunoallergology Unit, Careggi University Hospital, Florence, Italy.
- 92Clinic for Pulmonary Diseases, Clinical Center of Serbia, Faculty of Medicine, University of Belgrade, Serbian Association for Asthma and COPD, Belgrade, Serbia.
- 93Allergy Center, CUF Descobertas Hospital, Lisbon, Portugal.
- 94Rhinology Unit & Smell Clinic, ENT Department, Hospital Clínic, Barcelona, Spain.
- 95Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, CIBERES, University of Barcelona, Barcelona, Spain.
- 96Pediatrics and Child Health Research Institute, Central Clinical Hospital of the Russian Academy of Sciences, Russian National Research Medical University, Moscow, Russia.
- 97Director of Center of Allergy, Immunology and Respiratory Diseases, Santa Fe, Argentina.
- 98Hospital of the Hospitaller Brothers in Buda, Budapest, Hungary.
- 99Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland.
- 100EFA European Federation of Allergy and Airways Diseases Patients' Associations, Brussels, Belgium.
- 101Department of Allergy, Immunology and Respiratory Medicine, Central Clinical School, Monash University, Alfred Health, Melbourne, Vic., Australia.
- 102Department of Otorhinolaryngology, Chiba University Hospital, Chiba, Japan.
- 103Department of Infection and Immunity, Luxembourg Institute of Health, Esch-sur-Alzette, Luxembourg.
- 104Department of Dermatology and Allergy Center, Odense Research Center for Anaphylaxis, Odense University Hospital, University of Southern Denmark, Odense, Denmark.
- 105Department of Biochemistry and Molecular Biology, School of Chemistry, Complutense University of Madrid, Madrid, Spain.
- 106Allergy Department, 2nd Pediatric Clinic, Athens General Children's Hospital "P&A Kyriakou", University of Athens, Athens, Greece.
- 107Department of Immunology and Allergology, Faculty of Medicine and Faculty Hospital in Pilsen, Charles University in Prague, Pilsen, Czech Republic.
- 108Allergy and Respiratory Diseases, Ospedale Policlino San Martino -University of Genoa, Genoa, Italy.
- 109Division of Allergy and Clinical Immunology, Department of Medicine, Agency of Health ASL Salerno, "Santa Maria della Speranza" Hospital, Salerno, Italy.
- 110Department of Internal Medicine and Paediatrics, Section of Geriatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium.
- 111Department of Otorhinolaryngology, Head and Neck Surgery, Section of Rhinology and Allergy, University Hospital Marburg, Philipps-Universität Marburg, Marburg, Germany.
- 112Ecole polytechnique Palaiseau, IRBA (Institut de Recherche bio-Médicale des Armées), Bretigny, France.
- 113Children's Hospital Srebrnjak, Zagreb, Croatia.
- 114School of Medicine, University J.J. Strossmayer, Osijek, Croatia.
- 115University Hospital, Sv Ivan Rilski', Sofia, Bulgaria.
- 116Asian Hospital and Medical Center, Manilla, Philippines.
- 117Allergy and Clinical Immunology Unit, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal.
- 118Institute of Immunology, Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
- 119Faculty of Medicine, ICBR - Coimbra Institute for Clinical and Biomedical Research, CIBB, University of Coimbra, Coimbra, Portugal.
- 120Medical University of Graz, Graz, Austria.
- 121Gérontopôle de Toulouse, INSERM 1027, Toulouse, France.
- 122Oasi Research Institute-IRCCS, Troina, Italy.
- 123Fondazione Mediterranea GB Morgagni, Catania, Italy.
- 124Allergy Unit, Hospital Regional Universitario de Malaga, Malaga, Spain.
- 125Allergy Research Group, Instituto de Investigación Biomedica de Malaga-IBIMA and ARADyAL, Malaga, Spain.
- 126Division of Allergy Asthma and Clinical Immunology, Emek Medical Center, Afula, Israel.
- 127Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel.
- 128Department of Otolaryngology-Head and Neck Surgery, Eye and Ear University Hospital, Beirut, Lebanon.
- 129Société Française de Gériatrie et Gérontologie, Paris, France.
- 130Department of Prevention of Environmental Hazards, Allergology & Immunology, Medical University of Warsaw, Warsaw, Poland.
- 131Department of Women and Children's Health (Paediatric Allergy), School of Life Course Sciences, Faculty of Life Sciences and Medicine, King's College London, London, UK.
- 132Peter Gorer Department of Immunobiology, School of Immunology and Microbial Sciences, Faculty of Life Sciences and Medicine, King's College London, London, UK.
- 133Children's Allergy Service, Evelina London Children's Hospital Guy'sand St Thomas, Hospital, London, UK.
- 134Asthma UK Centre for Allergic Mechanisms in Asthma, London, UK.
- 135Asthma Reference Center, School of Medicine of Santa Casa de Misericórdia of Vitória, Espírito Santo, Brazil.
- 136Faculty of Medicine, Fundacion Jimenez Diaz, CIBERES, Autonoma University of Madrid, Madrid, Spain.
- 137Department of Health Services Research, Department of Family Medicine Caphri - Care and Public Health Research Institute, Maastricht University, Maastrich, NL, USA.
- 138PROMISE Department, University of Palermo, Palermo, Italy.
- 139Department of Immunology, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic.
- 140Immunomodulation and Tolerance Group, Imperial College London, London, UK.
- 141Allergy and Clinical Immunology, Imperial College London, London, UK.
- 142Usher Institute, The University of Edinburgh, Edinburgh, UK.
- 143Royal Brompton and Harefield NHS Foundation Trust, London, UK.
- 144Department of Clinical Immunology, Wroclaw Medical University, Wroclaw, Poland.
- 145ALL-MED" Medical Research Institute, Wroclaw, Poland.
- 146Christine Kühne - Center for Allergy Research and Education, CK-CARE, Davos, Switzerland.
- 147MEDCIDS - Department of Community Medicine, Information and Health Decision Sciences, Faculty of Medicine, University of Porto, Porto, Portugal.
- 148Department of Respiratory Medicine, University Hospital Olomouc, Olomouc, Czech Republic.
- 149Pulmonary Division, Heart Institute (InCor, Hospital da Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil.
- 150Department of Dermatology and Venerology, Medical University of Graz, Graz, Austria.
- 151Austria Outpatient Allergy Clinic Reumannplatz, Vienna, Austria.
- 152Department of Respiratory Medicine, Copenhagen University Hospital-Hvidovre, Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
- 153Imunoalergologia, Centro Hospitalar Universitário de Coimbra and Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
- 154Health Planning Unit, Department of Social Medicine, Faculty of Medicine, University of Crete, Crete, Greece.
- 155International Primary Care Respiratory Group IPCRG, Aberdeen, UK.
- 156Allergy Unit, Málaga Regional University Hospital-IBIMA, Málaga, Spain.
- 157Federal University of Pampa, Uruguaiana, Brazil.
- 158Faculty of Medicine, Vilnius University, Institute of Clinical Medicine & Institute of Health Sciences, Vilnius, Lithuania.
- 159European Academy of Paediatrics (EAP/UEMS-SP, Brussels, Belgium.
- 160Aix-Marseille University, IRD, APHM, MEPHI, Marseille, France.
- 161IHU Méditerranée Infection, Marseille and IDESP, INSERM, University of Montpellier, Montpellier, France.
- 162Nova Southeastern University, Fort Lauderdale, FL, USA.
- 163Department of Occupational Diseases and Environmental Health, Nofer Institute of Occupational Medicine, Lodz, Poland.
- 164Department of Otolaryngology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
- 165Department of Pulmonary Diseases, Faculty of Medicine, Celal Bayar University, Manisa, Turkey.
- 166The Allergy and Asthma Institute, Islamabad, Pakistan.
- 167Universidad Católica de Córdoba, Universidad Nacional de Villa Maria, Villa Maria, Argentina.
- 168University Clinic of Respiratory and Allergic Diseases, Golnik, Slovenia.
- 169School of Medicine, University CEU San Pablo, Madrid, Spain.
- 170Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Davos, Switzerland.
- 171Department of Otolaryngology, Head and Neck Surgery, Universitätsmedizin Mainz, Mainz, Germany.
- 172Center for Rhinology and Allergology, Wiesbaden, Germany.
Abstract
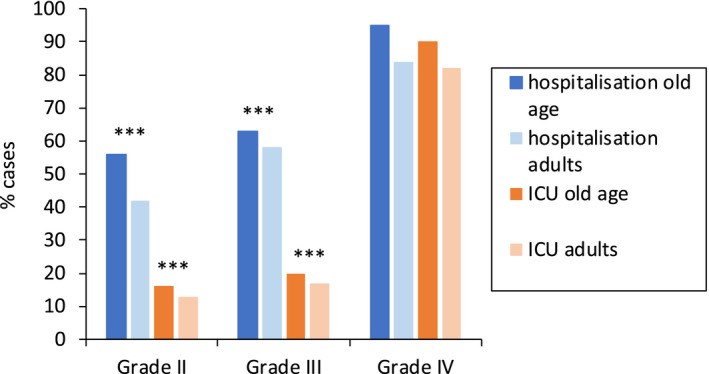
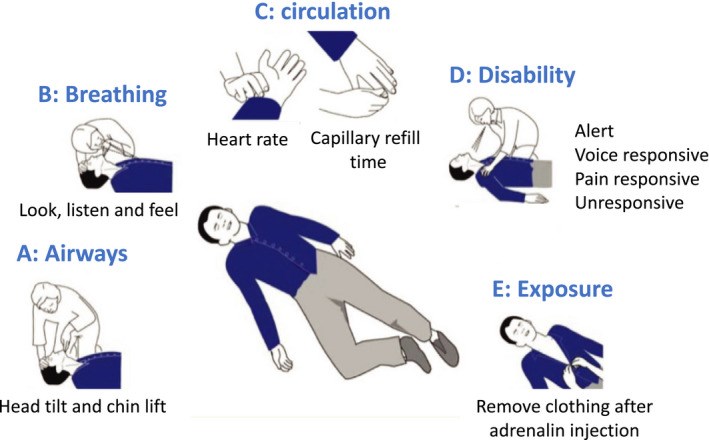
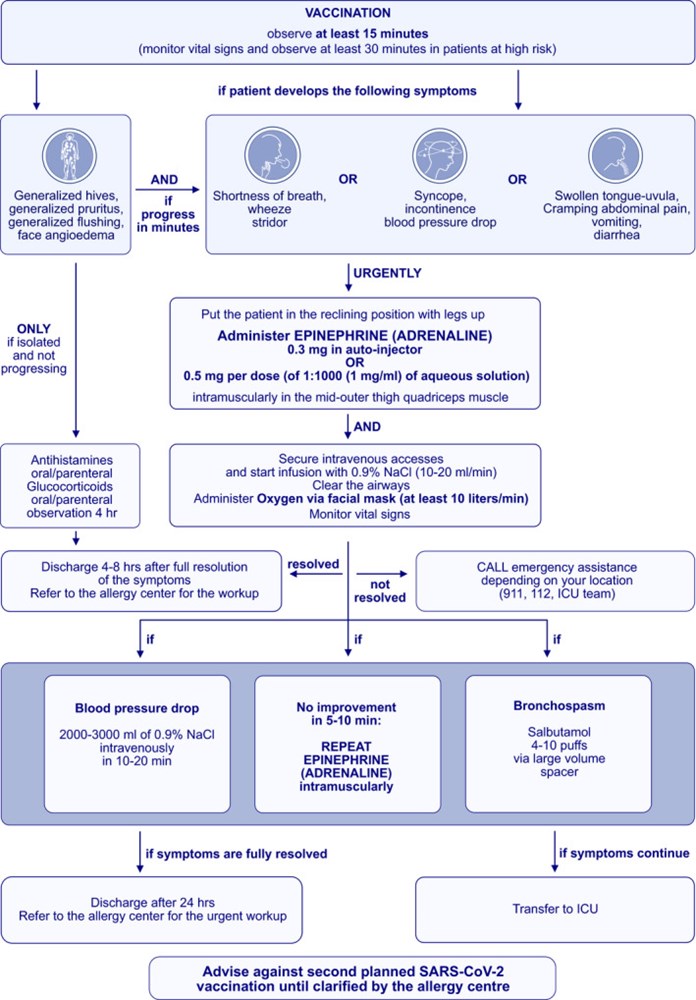
Older adults, especially men and/or those with diabetes, hypertension, and/or obesity, are prone to severe COVID-19. In some countries, older adults, particularly those residing in nursing homes, have been prioritized to receive COVID-19 vaccines due to high risk of death. In very rare instances, the COVID-19 vaccines can induce anaphylaxis, and the management of anaphylaxis in older people should be considered carefully. An ARIA-EAACI-EuGMS (Allergic Rhinitis and its Impact on Asthma, European Academy of Allergy and Clinical Immunology, and European Geriatric Medicine Society) Working Group has proposed some recommendations for older adults receiving the COVID-19 vaccines. Anaphylaxis to COVID-19 vaccines is extremely rare (from 1 per 100,000 to 5 per million injections). Symptoms are similar in younger and older adults but they tend to be more severe in the older patients. Adrenaline is the mainstay treatment and should be readily available. A flowchart is proposed to manage anaphylaxis in the older patients.
Keywords: COVID-19 vaccines; adrenaline; anaphylaxis; older (adults/people).
Conflict of interest statement
IA reports is Associate Editor Allergy and CTA. JB reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi‐Aventis, Takeda, Teva, Uriach, other from KYomed‐Innov, personal fees from Purina. VC reports personal fees from ALK, Allergy Therapeutics, LETI, Thermo Fisher, Merck, Astrazeneca, GSK. AC reports personal fees from BMS, MSD. ME reports personal fees from DBV Technologies, Mylan. MFR reports grants from ISCII (Spanish Government), Aimmune, Diater, personal fees from Aimmune, DBV, Novartis, SPRIM, ALK, Allergy Therapeutics, Diater, GSK, Thermo Fisher. BG reports grants from Astrazeneca, Novartis, MSD, Deva, Abdi Ibrahim, GSK. TH reports personal fees from GSK, Mundipharma, OrionPharma, Sanofi. LK reports grants and personal fees from Allergopharma, LETI Pharma, MEDA/Mylan, Sanofi, personal fees from HAL Allergie, Allergy Therapeut., Cassella med, grants from ALK Abelló, Stallergenes, Quintiles ASIT biotech, Lofarma, AstraZeneca, GSK, Inmunotk, and Membership: AeDA, DGHNO, Deutsche Akademie fürAllergologie und klinischeImmunologie, HNO‐BV, GPA, EAACI. PK reports personal fees from Adamed, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Hal Allergy, Lekam, Mylan, GSK, Novartis, Polpharma, Sanofi, from Teva. VK reports non‐financial support from AstraZeneca, DIMUNA, BerlinCHemieMenarini Baltic. RL reports grants from Astra Zeneca, Chiesi, GSK, other from Astra Zeneca, Novartis, GSK, Sanofi. JM reports personal fees and other from SANOFI GENZYME & REGENERON, NOVARTIS, ALLAKOS, grants and personal fees from MYLAN Pharma, URIACH Group, personal fees from Mitsubishi‐Tanabe, Menarini, UCB, AstraZeneca, GSK, MSD. MO reports grants from Astra Zeneca, Chiesi, GSK, DBV Technologies, Aimmune, Novartis, Pfizer, Regeneron, Sanofi, Boehringer Ingelheim; and volunteer President of the European Federation of Allergy and Airways Diseases Patients’ Associations EFA who receives unrestricted grants from pharmaceutical companies. Second Vice President of the Swedish Asthma and Allergy Association, who receives no income from companies. Takes part in Novartis Asthma Patient Advisory Committee, GSK Global Respiratory Patient Advisory Group and have participated/presented in AstraZeneca events. Whenever there was a honorarium, this went to EFA. OP reports research grants from Inmunotek S.L., Novartis, and MINECO. fees for giving scientific lectures or participation in Advisory Boards from Allergy Therapeutics, Amgen, AstraZeneca, Diater, GlaxoSmithKline, S.A, Inmunotek S.L, Novartis, Sanofi Genzyme and Stallergenes. NP reports personal fees from Novartis, Nutricia, HAL, MENARINI/FAES FARMA, SANOFI, MYLAN/MEDA, BIOMAY, AstraZeneca, GSK, MSD, ASIT BIOTECH, Boehringer Ingelheim, grants from Gerolymatos International SA, Capricare. OP reports grants and personal fees from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, BencardAllergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., rom Laboratorios LETI/LETI Pharma, Anergis S.A., Glaxo Smith Kline, personal fees from Astellas Pharma Global, MEDA Pharma/MYLAN, EUFOREA, ROXALL Medizin, Novartis, Sanofi‐Aventis, and Sanofi Genzyme, Med Update Europe GmbH, streamedup! GmbH, John Wiley and Sons, AS, Paul‐Martini‐Stiftung (PMS), Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies grants from Pohl‐Boskamp, Inmunotek S.L., Biomay, Circassia. DP reports grants and personal fees from GlaxoSmithKline, personal fees and non‐financial support from Boehringer Ingelheim, personal fees from Belupo, AbbVie, MSD, Chiesi, Menarini, Pliva, Revenio, non‐financial support from Philips. YR reports grants from BIOPHYTIS, NOVARTIS. BS reports personal fees from Allergopharma, Viatris, TEVA, ADAMED, patient ombudsman, Polish Allergology Society grants from AstraZeneca, National Health Programm, grant and personal fees from Polpharma, AstraZeneca. AS reports grants and personal fees from Medical Research Council, Thermo Fisher, Buhlmann, Infomed, Nutricia and Nestle, Allergy Therapeutics, Novartis and Stallergenes, grants from Food Allergy Research and Education, Asthma UK, NIAID / Immune Tolerance Network, non‐financial support from National Institute for Health Research, Thermo Fisher and Buhlmann. Dr. Serpa reports personal fees from Takeda, personal fees and other from Novartis, personal fees from Sanofi, personal fees from GSK, other from Astra Zeneca. JS reports grants and personal fees from SANOFI, personal fees from GSK, NOVARTIS, ASTRA ZENECA, MUNDIPHARMA, FAES FARMA. AS reports grants from HDRUK. MS reports grants from Swiss National Science Foundation (SNF), GlaxoSmithKline (GSK). RS reports grants from São Paulo Research Foundation, MSD, grants and personal fees from Novartis, grants, personal fees and non‐financial support from AstraZeneca, grants, personal fees and non‐financial support from Chiesi, personal fees and non‐financial support from Boehringer Ingelheim. GS reports grants and personal fees from ALK‐Abello, personal fees from Novartis, Bencard, Stallergens, HAL, Allergopharma, Mylan. AMTB reports grants and personal fees from Teva, AstraZeneca, GSK (GlaxoSmithKline), Sanofi, Mundipharma, personal fees from Bial, grants from Leti, Novartis. STS reports personal fees from AstraZeneca, ERT, Novartis, Sanofi Pharma, Roche Products, grants from GSK. MT reports grants from European Commission, SEAIC, ISCIII, personal fees from Diater laboratory, Leti laboratory, Aimmune Therapeutics. IT reports personal fees from Honoraria for educational activities, speaking engagements, advisory boards from Boehringer Ingelheim, Astra Zeneca, GSK, Novartis and grants from GSK Hellas and Elpen. CSU reports personal fees from Astra Zeneca, personal fees from Chiesi, grants and personal fees from Novartis, Boehringer Ingelheim, personal fees from ALK‐Abello, TEVA, Orion Pharma, grants Sanofi Genzyme, personal fees and non‐financial support from GSK. IV reports personal fees from Novartis, Sanofi, personal fees and non‐financial support from Thermo Fisher, non‐financial support from Beckman Coulter. DW reports other from Kaleo, Mylan, and on the AAAAI/ACAAI Joint Task Force on Practice Parameters updating the Anaphylaxis practice parameter. SW reports other from Pfizer, Kaleo, Bausch Lomb. MW reports other from Regeneron Pharmaceuticals, DBV Technologies S.A, Stallergenes GmbH, HAL Allergie GmbH, BencardAllergie GmbH, Allergopharma GmbH & Co. KG, ALK‐AbellóArzneimittel GmbH, Mylan Germany GmbH, Leo Pharma GmbH, Sanofi‐Aventis Deutschland GmbH, Aimmune Therapeutics UK Limited, Actelion Pharmaceuticals Deutschland GmbH, Novartis AG, Biotest AG, AbbVie Deutschland GmbH & Co. KG, Lilly Deutschland GmbH. TZ reports and Organizational affiliations: Committee member: WHO‐Initiative "Allergic Rhinitis and Its Impact on Asthma" (ARIA), Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI), Board Chairman: European Centre for Allergy Research Foundation (ECARF). President: Global Allergy and Asthma European Network (GA2LEN), Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organization (WAO).
Figures
Similar articles
Klimek L, Jutel M, Akdis CA, Bousquet J, Akdis M, Torres MJ, Agache I, Canonica GW, Del Giacco S, O'Mahony L, Shamji MH, Schwarze J, Untersmayr E, Ring J, Bedbrook A, Worm M, Zuberbier T, Knol E, Hoffmann-Sommergruber K, Chivato T.Allergy. 2021 Jun;76(6):1624-1628. doi: 10.1111/all.14726.PMID: 33378789
Recent developments in the immunopathology of COVID-19.
Zhang HP, Sun YL, Wang YF, Yazici D, Azkur D, Ogulur I, Azkur AK, Yang ZW, Chen XX, Zhang AZ, Hu JQ, Liu GH, Akdis M, Akdis CA, Gao YD.Allergy. 2023 Feb;78(2):369-388. doi: 10.1111/all.15593. Epub 2022 Dec 5.PMID: 36420736 Free PMC article. Review.
Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper.
Barbaud A, Garvey LH, Arcolaci A, Brockow K, Mori F, Mayorga C, Bonadonna P, Atanaskovic-Markovic M, Moral L, Zanoni G, Pagani M, Soria A, Jošt M, Caubet JC, Carmo A, Mona AA, Alvarez-Perea A, Bavbek S, Benedetta B, Bilo MB, Blanca-López N, Bogas HG, Buonomo A, Calogiuri G, Carli G, Cernadas J, Cortellini G, Celik G, Demir S, Doña I, Dursun AB, Eberlein B, Faria E, Fernandes B, Garcez T, Garcia-Nunez I, Gawlik R, Gelincik A, Gomes E, Gooi JHC, Grosber M, Gülen T, Hacard F, Hoarau C, Janson C, Johnston SL, Joerg L, Kepil Özdemir S, Klimek L, Košnik M, Kowalski ML, Kuyucu S, Kvedariene V, Laguna JJ, Lombardo C, Marinho S, Merk H, Meucci E, Morisset M, Munoz-Cano R, Murzilli F, Nakonechna A, Popescu FD, Porebski G, Radice A, Regateiro FS, Röckmann H, Romano A, Sargur R, Sastre J, Scherer Hofmeier K, Sedláčková L, Sobotkova M, Terreehorst I, Treudler R, Walusiak-Skorupa J, Wedi B, Wöhrl S, Zidarn M, Zuberbier T, Agache I, Torres MJ.Allergy. 2022 Aug;77(8):2292-2312. doi: 10.1111/all.15241. Epub 2022 Mar 5.PMID: 35112371
Asano K, Fujisawa T, Nakamura Y, Nagata M, Hide M, Fujieda S.Arerugi. 2021;70(3):215-223. doi: 10.15036/arerugi.70.215.PMID: 34011777 Japanese. No abstract available.
Anaphylaxis in elderly people.
Ventura MT, Boni E, Taborda-Barata L, Blain H, Bousquet J.Curr Opin Allergy Clin Immunol. 2022 Dec 1;22(6):435-440. doi: 10.1097/ACI.0000000000000855. Epub 2022 Sep 14.PMID: 36165408 Review.
Cited by
Haq HN, Khan H, Chaudhry H, Nimmala S, Demidovich J, Papudesi BN, Potluri SD.J Natl Med Assoc. 2022 Dec;114(6):601-612. doi: 10.1016/j.jnma.2022.08.003. Epub 2022 Oct 28.PMID: 36511275 Free PMC article. Review.
Xiang LL, Wan QQ, Wang YM, He SJ, Xu WJ, Ding M, Zhang JJ, Sun YL, Dong X, Zhou Y, Cui YB, Gao YD.J Asthma Allergy. 2022 Sep 7;15:1245-1261. doi: 10.2147/JAA.S360381. eCollection 2022.PMID: 36101840 Free PMC article.
COVID-19 vaccine side effects among nursing home residents and staff.
Bhatnagar S, Jones K, Montoya A.J Med Virol. 2022 Aug;94(8):3491-3493. doi: 10.1002/jmv.27756. Epub 2022 Apr 12.PMID: 35365909 Free PMC article. No abstract available.
Recent advances and developments in COVID-19 in the context of allergic diseases.
Ding M, Dong X, Sun YL, Sokolowska M, Akdis M, van de Veen W, Azkur AK, Azkur D, Akdis CA, Gao YD.Clin Transl Allergy. 2021 Sep;11(7):e12065. doi: 10.1002/clt2.12065.PMID: 34582102 Free PMC article.
Klimek L, Pfaar O, Hamelmann E, Kleine-Tebbe J, Taube C, Wagenmann M, Werfel T, Brehler R, Novak N, Mülleneisen N, Becker S, Worm M.Allergol Select. 2021 Aug 24;5:251-259. doi: 10.5414/ALX02245E. eCollection 2021.PMID: 34533543 Free PMC article.
KMEL References
References
-
- Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID‐19 patients: results of the SARS‐RAS study of the Italian Society of Hypertension. Hypertension 2020;76(2):366‐372. - PubMed
-
- Telford CT, Onwubiko U, Holland D, et al. Mass screening for SARS‐CoV‐2 infection among residents and staff in twenty‐eight long‐term care facilities in Fulton County, Georgia. medRxiv 2020. 10.1101/2020.07.01.20144162 - DOI
-
- Grabowski DC, Mor V. Nursing home care in crisis in the wake of COVID‐19. JAMA 2020;324(1):23. - PubMed
-
- Klimek L, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on severe allergic reactions to COVID‐19 vaccines ‐ an EAACI‐ARIA position paper. Allergy. 2021;76(6):1624‐1628. - PubMed
-
- Ruggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675‐5684. - PubMed
-
- Shimabukuro T. COVID‐19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP) January 27, 2021. National Center for Immunization and respiratory diseases CDC https://wwwcdcgov/vaccines/acip/meetings/downloads/slides‐2021‐01/06‐COVID‐Shimabukuropdf. 2021.
-
- Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID‐19 vaccine: a potential role of Polyethylene glycol? Allergy 2021;76(6):1617‐1618. - PubMed
-
- Campbell RL, Hagan JB, Li JT, et al. Anaphylaxis in emergency department patients 50 or 65 years or older. Ann Allergy Asthma Immunol. 2011;106(5):401‐406. - PubMed
-
- Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1(8009):466‐469. - PubMed
-
- Niggemann B, Beyer K. Time for a new grading system for allergic reactions? Allergy. 2016;71(2):135‐136. - PubMed
-
- Muraro A, Fernandez‐Rivas M, Beyer K, et al. The urgent need for a harmonized severity scoring system for acute allergic reactions. Allergy. 2018;73(9):1792‐1800. - PubMed
-
- Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63(2):226‐232. - PubMed
-
- Dolle‐Bierke S, Siebenhaar F, Burmeister T, Worm M. Detection of KIT D816V mutation in patients with severe anaphylaxis and normal basal tryptase‐first data from the Anaphylaxis Registry (NORA). J Allergy Clin Immunol. 2019;144(5):1448‐1450.e1. - PubMed
-
- Worm M, Francuzik W, Renaudin JM, et al. Factors increasing the risk for a severe reaction in anaphylaxis: an analysis of data from The European Anaphylaxis Registry. Allergy. 2018;73(6):1322‐1330. - PubMed
-
- Bilo MB, Martini M, Tontini C, Corsi A, Antonicelli L. Anaphylaxis. Eur Ann Allergy Clin Immunol. 2020;53(01):4. - PubMed
-
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy. 2015;45(8):1288‐1295. - PubMed
-
- Tejedor‐Alonso MA, Farias‐Aquino E, Perez‐Fernandez E, Grifol‐Clar E, Moro‐Moro M, Rosado‐Ingelmo A. Relationship between anaphylaxis and use of beta‐blockers and angiotensin‐converting enzyme inhibitors: a systematic review and meta‐analysis of observational studies. J Allergy Clin Immunol Pract. 2019;7(3):879‐897.e5. - PubMed
-
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69(8):1026‐1045. - PubMed
-
- Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010;10(4):354‐361. - PubMed
-
- Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76‐80. - PubMed
-
- Bilo MB, Cichocka‐Jarosz E, Pumphrey R, et al. Self‐medication of anaphylactic reactions due to Hymenoptera stings‐an EAACI Task Force Consensus Statement. Allergy. 2016;71(7):931‐943. - PubMed
-
- O'Brien ME, Koehl JL, Raja AS, Erickson TB, Hayes BD. Age‐related cardiovascular outcomes in older adults receiving epinephrine for anaphylaxis in the emergency department. J Allergy Clin Immunol Pract. 2019;7(8):2888‐2890. - PubMed
-
- Rukma P. Glucagon for refractory anaphylaxis. Am J Ther. 2019;26(6):e755‐e756. - PubMed
-
- McLure M, Eastwood K, Parr M, Bray J. A Rapid review of advanced life support guidelines for cardiac arrest associated with anaphylaxis. Resuscitation 2021;159:137–149. - PubMed
-
- Lieberman PL. Recognition and first‐line treatment of anaphylaxis. Am J Med. 2014;127(1 Suppl):S6‐11. - PubMed
-
- Ring J, Beyer K, Biedermann T, et al. Guideline for acute therapy and management of anaphylaxis: S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB). Allergo J Int. 2014;23(3):96‐112. - PMC - PubMed