Allergen immunotherapy in MASK-air users in real-life: Results of a Bayesian mixed-effects model
Bernardo Sousa-Pinto 1 2, Luís Filipe Azevedo 1 2, Ana Sá-Sousa 1 2, Rafael José Vieira 1 2, Rita Amaral 1 2, Ludger Klimek 3 4, Wienczyslawa Czarlewski 5 6, Josep M Anto 7 8 9 10, Anna Bedbrook 6, Violeta Kvedariene 11 12, Maria Teresa Ventura 13, Ignacio J Ansotegui 14, Karl-Christian Bergmann 15 16 17, Luisa Brussino 18, G Walter Canonica 19 20, Victoria Cardona 21, Pedro Carreiro-Martins 22 23, Thomas Casale 24, Lorenzo Cecchi 25, Tomás Chivato 26, Derek K Chu 27, Cemal Cingi 28, Elisio M Costa 29, Alvaro A Cruz 30 31, Giulia De Feo 32, Philippe Devillier 33, Wytske J Fokkens 34, Mina Gaga 35, Bilun Gemicioğlu 36, Tari Haahtela 37, Juan Carlos Ivancevich 38, Zhanat Ispayeva 39, Marek Jutel 40 41, Piotr Kuna 42, Igor Kaidashev 43, Helga Kraxner 44, Désirée E Larenas-Linnemann 45, Daniel Laune 46, Brian Lipworth 47, Renaud Louis 48 49, Michaël Makris 50, Riccardo Monti 51, Mario Morais-Almeida 52, Ralph Mösges 53 54, Joaquim Mullol 55 56, Mikaëla Odemyr 57, Yoshitaka Okamoto 58, Nikolaos G Papadopoulos 59, Vincenzo Patella 60, Nhân Pham-Thi 61, Frederico S Regateiro 1 2, Sietze Reitsma 34, Philip W Rouadi 62 63, Boleslaw Samolinski 64, Milan Sova 65, Ana Todo-Bom 1, Luis Taborda-Barata 66 67 68, Peter Valentin Tomazic 69, Sanna Toppila-Salmi 37, Joaquin Sastre 70, Ioanna Tsiligianni 71 72, Arunas Valiulis 73, Dana Wallace 74, Susan Waserman 75, Arzu Yorgancioglu 76, Mihaela Zidarn 77 78, Torsten Zuberbier 15 16 17 79, João Almeida Fonseca 1 2 80, Jean Bousquet 15 16 17 78 81, Oliver Pfaar 82
Affiliations
Affiliations
- MEDCIDS-Department of Community Medicine, Information and Health Decision Sciences, Faculty of Medicine, University of Porto, Porto, Portugal.
- CINTESIS-Center for Health Technology and Services Research, University of Porto, Porto, Portugal.
- Department of Otolaryngology, Head and Neck Surgery, Universitätsmedizin Mainz, Mainz, Germany.
- Center for Rhinology and Allergology, Wiesbaden, Germany.
- Medical Consulting Czarlewski, Levallois, France.
- MASK-air, Montpellier, France.
- ISGlobal, Barcelona Institute for Global Health, Barcelona, Spain.
- IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain.
- Universitat Pompeu Fabra (UPF), Barcelona, Spain.
- CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain.
- Institute of Biomedical Sciences, Department of Pathology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
- Institute of Clinical Medicine, Clinic of Chest Diseases and Allergology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
- University of Bari Medical School, Unit of Geriatric Immunoallergology, Bari, Italy.
- Department of Allergy and Immunology, Hospital Quirónsalud Bizkaia, Bilbao, Spain.
- Institute of Allergology, Charité-Universitätsmedizin Berlin, Berlin, Germany.
- Corporate Member of Freie Universität Berlin, Berlin, Germany.
- Humboldt-Universität zu Berlin, Berlin, Germany.
- Department of Medical Sciences, Allergy and Clinical Immunology Unit, University of Torino & Mauriziano Hospital, Torino, Italy.
- Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy.
- Personalized Medicine, Asthma and Allergy, Humanitas Clinical and Research Center IRCCS, Rozzano, Italy.
- Allergy Section, Department of Internal Medicine, Hospital Vall d'Hebron & ARADyAL Research Network, Barcelona, Spain.
- Serviço de Imunoalergologia, Hospital de Dona Estefânia, Centro Hospitalar Universitário de Lisboa Central, Lisbon, Portugal.
- NOVA Medical School/Comprehensive Health Research Centre (CHRC), Lisbon, Portugal.
- Division of Allergy/immunology, University of South Florida, Tampa, Florida, USA.
- SOS Allergology and Clinical Immunology, USL Toscana Centro, Prato, Italy.
- School of Medicine, University CEU San Pablo, Madrid, Spain.
- Department of Medicine and Health Research Methods, Evidence & Impact, McMaster University, Hamilton, Ontario, Canada.
- Eskisehir Osmangazi University, Medical Faculty, ENT Department, Eskisehir, Turkey.
- UCIBIO, REQUINTE, Faculty of Pharmacy and Competence Center on Active and Healthy Ageing of University of Porto (Porto4Ageing), Porto, Portugal.
- Fundaçao ProAR, Federal University of Bahia, Salvador, Bahia, Brazil.
- GARD/WHO Planning Group, Salvador, Bahia, Brazil.
- Department of Medicine, Surgery and Dentistry 'Scuola Medica Salernitana', University of Salerno, Salerno, Italy.
- VIM Suresnes, UMR_0892, Pôle des Maladies des Voies Respiratoires, Hôpital Foch, Université Paris-Saclay, Suresnes, France.
- Department of Otorhinolaryngology, Amsterdam University Medical Centres, location AMC, Amsterdam, The Netherlands.
- ERS President 2017-2018, Athens Chest Hospital, 7th Respiratory Medicine Department and Asthma Center, Athens, Greece.
- Department of Pulmonary Diseases, Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Istanbul, Turkey.
- Skin and Allergy Hospital, Helsinki University Hospital, University of Helsinki, Helsinki, Finland.
- Servicio de Alergia e Immunologia, Clinica Santa Isabel, Buenos Aires, Argentina.
- Department of Allergology and Clinical Immunology of the Kazakh National Medical University, Almaty, Kazakhstan.
- Department of Clinical Immunology, Wrocław Medical University, Wroclaw, Poland.
- ALL-MED Medical Research Institute, Wroclaw, Poland.
- Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, Lodz, Poland.
- Poltava State Medical University, Poltava, Ukraine.
- Department of Otorhinolaryngology, Head and Neck Surgery, Semmelweis University, Budapest, Hungary.
- Center of Excellence in Asthma and Allergy, Médica Sur Clinical Foundation and Hospital, México City, Mexico.
- KYomed INNOV, Montpellier, France.
- Scottish Centre for Respiratory Research, Cardiovascular & Diabetes Medicine, Medical Research Institute, Ninewells Hospital, University of Dundee, Dundee, UK.
- Department of Pulmonary Medicine, CHU Sart-Tilman, Liege, Belgium.
- GIGA I3 Research Group, Liege, Belgium.
- Allergy Unit 'D Kalogeromitros', 2nd Department of Dermatology and Venereology, National & Kapodistrian University of Athens, 'Attikon' University Hospital, Chaidari, Greece.
- Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario A Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
- Allergy Center, CUF Descobertas Hospital, Lisbon, Portugal.
- ClinCompetence Cologne GmbH, Cologne, Germany.
- IMSB, Medical Faculty, University of Cologne, Cologne, Germany.
- Rhinology Unit & Smell Clinic, ENT Department, Hospital Clínic, Barcelona, Spain.
- Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, CIBERES, University of Barcelona, Barcelona, Spain.
- EFA European Federation of Allergy and Airways Diseases Patients' Associations, Brussels, Belgium.
- Department of Otorhinolaryngology, Chiba University Hospital, Chiba, Japan.
- Allergy Department, 2nd Pediatric Clinic, University of Athens, Athens, Greece.
- Division of Allergy and Clinical Immunology, Department of Medicine, Agency of Health ASL Salerno, 'Santa Maria della Speranza' Hospital, Salerno, Italy.
- Ecole Polytechnique Palaiseau, IRBA (Institut de Recherche bio-Médicale des Armées), Bretigny, France.
- Department of Otolaryngology-Head and Neck Surgery, Eye and Ear University Hospital, Beirut, Lebanon.
- ENT Department, Dar Al Shifa Hospital, Salmiya, Kuwait.
- Department of Prevention of Environmental Hazards, Allergology and Immunology, Medical University of Warsaw, Warsaw, Poland.
- Department of Pulmonary Medicine and Tuberculosis, University Hospital Brno, Liskovec, Czech Republic.
- Faculty of Health Sciences, University of Beira Interior, Covilhã, Portugal.
- UBIAir-Clinical & Experimental Lung Centre, University of Beira Interior, Covilhã, Portugal.
- Department of Immunoallergology, Cova da Beira University Hospital Centre, Covilhã, Portugal.
- Department of General ORL, H&NS, Medical University of Graz, ENT-University Hospital Graz, Graz, Austria.
- Fundacion Jimenez Diaz, CIBERES, Faculty of Medicine, Autonoma University of Madrid, Madrid, Spain.
- Health Planning Unit, Department of Social Medicine, Faculty of Medicine, University of Crete, Rethymno, Greece.
- International Primary Care Respiratory Group IPCRG, Aberdeen, Scotland.
- Institute of Clinical Medicine and Institute of Health Sciences, Medical Faculty of Vilnius University, Vilnius, Lithuania.
- Nova Southeastern University, Fort Lauderdale, Florida, USA.
- Department of Medicine, Clinical Immunology and Allergy, McMaster University, Hamilton, Ontario, Canada.
- Department of Pulmonology, Celal Bayar University, Manisa, Turkey.
- University Clinic of Respiratory and Allergic Diseases, Golnik, Slovenia.
- University of Ljubljana, Faculty of Medicine, Ljubljana, Slovenia.
- Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Allergology and Immunology, Berlin, Germany.
- Medicina, EDucação, I&D e Avaliação, Lda (MEDIDA), Porto, Portugal.
- University Hospital Montpellier, Montpellier, France.
- Department of Otorhinolaryngology, Head and Neck Surgery, Section of Rhinology and Allergy, University Hospital Marburg, Philipps-Universität Marburg, Marburg, Germany.
Abstract
Background: Evidence regarding the effectiveness of allergen immunotherapy (AIT) on allergic rhinitis has been provided mostly by randomised controlled trials, with little data from real-life studies.
Objective: To compare the reported control of allergic rhinitis symptoms in three groups of users of the MASK-air® app: those receiving sublingual AIT (SLIT), those receiving subcutaneous AIT (SCIT), and those receiving no AIT.
Methods: We assessed the MASK-air® data of European users with self-reported grass pollen allergy, comparing the data reported by patients receiving SLIT, SCIT and no AIT. Outcome variables included the daily impact of allergy symptoms globally and on work (measured by visual analogue scales-VASs), and a combined symptom-medication score (CSMS). We applied Bayesian mixed-effects models, with clustering by patient, country and pollen season.
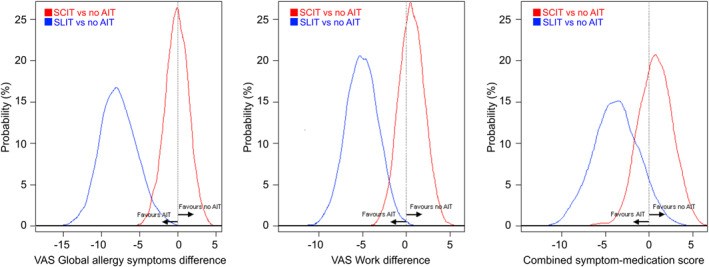
Results: We analysed a total of 42,756 days from 1,093 grass allergy patients, including 18,479 days of users under AIT. Compared to no AIT, SCIT was associated with similar VAS levels and CSMS. Compared to no AIT, SLIT-tablet was associated with lower values of VAS global allergy symptoms (average difference = 7.5 units out of 100; 95% credible interval [95%CrI] = -12.1;-2.8), lower VAS Work (average difference = 5.0; 95%CrI = -8.5;-1.5), and a lower CSMS (average difference = 3.7; 95%CrI = -9.3;2.2). When compared to SCIT, SLIT-tablet was associated with lower VAS global allergy symptoms (average difference = 10.2; 95%CrI = -17.2;-2.8), lower VAS Work (average difference = 7.8; 95%CrI = -15.1;0.2), and a lower CSMS (average difference = 9.3; 95%CrI = -18.5;0.2).
Conclusion: In patients with grass pollen allergy, SLIT-tablet, when compared to no AIT and to SCIT, is associated with lower reported symptom severity. Future longitudinal studies following internationally-harmonised standards for performing and reporting real-world data in AIT are needed to better understand its 'real-world' effectiveness.
Keywords: allergic rhinitis; immunotherapy; mobile health; patient-reported outcomes; real-life data analysis.
Conflict of interest statement
IA reports personal fees from Hikma, Roxall, Astra Zeneca, Menarini, UCB, Faes Farma, Sanofi, Mundipharma, Bial, Amgen, Stallergenes Greer, Bayer. JB reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi‐Aventis, Takeda, Teva, Uriach, other from KYomed‐Innov, personal fees from Purina, TC reports grants from Stallergenes Greer. AC reports personal fees from GSK, AstraZeneca, Sanofi, Novartis, Boehringer Ingelheim, Mylan, Mantecorp, Eurofarma. PhD reports personal fees and non‐financial support from Alk Abello, Stallergenes Greer. TH reports personal fees from GSK, Mundipharma, Orion Pharma, and Sanofi. JCI reports personal fees from Laboratorios Casasco, Abbott Ecuador, Bago Bolivia, Sanofi, Eurofarma Argentina, VK reports other from BerlinCHemie Menarini, other from Norameda. DLL reports personal fees from Allakos, Amstrong, Astrazeneca, DBV Technologies, Grunenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Alakos, Gossamer, Carnot, grants from Sanofi, Astrazeneca, Novartis, Circassia, UCB, GSK, Purina institute. RL reports grants and personal fees from GSK, AZ, Chiesi, Novartis, personal fees from Sanofi. MM reports personal fees from Menarini, personal fees from Astra Zeneca, personal fees from GSK, personal fees from Mylan, personal fees from Sanofi, personal fees from Pfizer, personal fees from Chiesi, RM reports personal fees from ALK, allergopharma, Allergy Therapeutics, Friulchem, Hexal, Servier, Klosterfrau, Bayer, FAES, GSK, MSD, Johnson&Johnson, Meda, Stada, UCB, Nuvo, Menarini, Mundipharma, Pohl‐Boskamp,grants from ASIT biotech, Leti, Optima, BitopAG, Hulka, Ursapharm, Inmunotek, grants and personal fees from Bencard, Stallergenes, grants, personal fees and non‐financial support from Lofarma, non‐financial support from Roxall, Atmos, Bionorica, Otonomy, Ferrero, personal fees and non‐financial support from Novartis. JM reports personal fees and other from SANOFI‐GENZYME & REGENERON, NOVARTIS, ALLAKOS, grants and personal fees from MYLAN Pharma, URIACH Group, personal fees from Mitsubishi‐Tanabe, Menarini, UCB, AstraZeneca, GSK, MSD. NGP reports personal fees from Novartis, Nutricia, HAL, MENARINI/FAES FARMA, SANOFI, MYLAN/MEDA, BIOMAY, AstraZeneca, GSK, MSD, ASIT BIOTECH, Boehringer Ingelheim, grants from Gerolymatos International SA, Capricare. OP reports grants and personal fees from ALK‐Abelló, Allergopharma, g Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, grants and personal fees from ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., GlaxoSmithKline, personal fees from Astellas Pharma Global, personal fees from EUFOREA, ROXALL Medizin, Novartis, Sanofi‐Aventis and Sanofi‐Genzyme, Med Update Europe GmbH, streamedup! GmbH, Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies, MEDA Pharma/MYLAN, John Wiley and Sons, AS, Paul‐Martini‐Stiftung (PMS), Ingress‐Health HWM, Regeneron Pharmaceuticals Inc., grants from Pohl‐Boskamp, Inmunotek S.L., Biomay, Circassia. JS reports grants and personal fees from SANOFI, personal fees from GSK, NOVARTIS, ASTRA ZENECA, MUNDIPHARMA, FAES FARMA. AMTB reports grants and personal fees from Novartis, Mundipharma, Teva Pharma, GSK (GlaxoSmithKline), AstraZeneca, grants from Leti, personal fees from BIAL. Dr. Tsiligianni reports personal fees from Honoraria for educational activities, speaking engagements, advisory boards from Boehringer Ingelheim, Astra Zeneca, GSK, Novartis, MSD and grants from GSK Hellas Astra Zeneca, and Elpen. DW reports personal fees from ALK.
Figures
Similar articles
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, Friedrichs F, Fuchs T, Hamelmann E, Hartwig-Bade D, Hering T, Huttegger I, Jung K, Klimek L, Kopp MV, Merk H, Rabe U, Saloga J, Schmid-Grendelmeier P, Schuster A, Schwerk N, Sitter H, Umpfenbach U, Wedi B, Wöhrl S, Worm M, Kleine-Tebbe J, Kaul S, Schwalfenberg A.Allergo J Int. 2014;23(8):282-319. doi: 10.1007/s40629-014-0032-2.PMID: 26120539 Free PMC article.
Vogelberg C, Brüggenjürgen B, Richter H, Jutel M.Patient Prefer Adherence. 2020 May 13;14:817-827. doi: 10.2147/PPA.S242957. eCollection 2020.PMID: 32494127 Free PMC article.
Contoli M, Porsbjerg C, Buchs S, Larsen JR, Freemantle N, Fritzsching B.J Allergy Clin Immunol. 2023 Mar 3:S0091-6749(23)00284-1. doi: 10.1016/j.jaci.2023.02.024. Online ahead of print.PMID: 36871918
Ohashi-Doi K, Lund K, Mitobe Y, Okamiya K.Biol Pharm Bull. 2020;43(1):41-48. doi: 10.1248/bpb.b19-00093.PMID: 31902930 Review.
Calderon MA, Casale TB, Nelson HS, Demoly P.J Allergy Clin Immunol. 2013 Dec;132(6):1322-36. doi: 10.1016/j.jaci.2013.09.004. Epub 2013 Oct 18.PMID: 24139829 Review.
Cited by
Real-life evidence in allergen immunotherapy: Moving forward with mHealth apps.
Sousa-Pinto B, Pfaar O, Bousquet J.Allergol Select. 2023 Mar 1;7:47-56. doi: 10.5414/ALX02343E. eCollection 2023.PMID: 36925994 Free PMC article. Review.
Yuriev S, Rodinkova V, Mokin V, Varchuk I, Sharikadze O, Marushko Y, Halushko B, Kurchenko A.Clin Mol Allergy. 2023 Feb 3;21(1):1. doi: 10.1186/s12948-022-00182-z.PMID: 36737770 Free PMC article.
Bousquet J, Anto JM, Sousa-Pinto B, Czarlewski W, Bedbrook A, Haahtela T, Klimek L, Pfaar O, Kuna P, Kupczyk M, Regateiro FS, Samolinski B, Valiulis A, Yorgancioglu A, Arnavielhe S, Basagaña X, Bergmann KC, Bosnic-Anticevich S, Brussino L, Canonica GW, Cardona V, Cecchi L, Chaves-Loureiro C, Costa E, Cruz AA, Gemicioglu B, Fokkens WJ, Ivancevich JC, Kraxner H, Kvedariene V, Larenas-Linnemann DE, Laune D, Louis R, Makris M, Maurer M, Melén E, Micheli Y, Morais-Almeida M, Mullol J, Niedoszytko M, Okamoto Y, Papadopoulos NG, Patella V, Pham-Thi N, Rouadi PW, Sastre J, Scichilone N, Sheikh A, Sofiev M, Taborda-Barata L, Toppila-Salmi S, Tsiligianni I, Valovirta E, Ventura MT, Vieira RJ, Zidarn M, Amaral R, Ansotegui IJ, Bédard A, Benveniste S, Bewick M, Bindslev-Jensen C, Blain H, Bonini M, Bourret R, Braido F, Carreiro-Martins P, Charpin D, Cherrez-Ojeda I, Chivato T, Chu DK, Cingi C, Del Giacco S, de Blay F, Devillier P, De Vries G, Doulaptsi M, Doyen V, Dray G, Fontaine JF, Gomez RM, Hagemann J, Heffler E, Hofmann M, Jassem E, Jutel M, Keil T, Kritikos V, Kull I, Kulus M, Lourenço O, Mathieu-Dupas E, Menditto E, Mösges R, Murray R, Nadif R, Neffen H, Nicola S, O'Hehir R, Olze H, Pa…See abstract for full author list ➔Clin Transl Allergy. 2023 Jan;13(1):e12215. doi: 10.1002/clt2.12215.PMID: 36705508 Free PMC article.
Real-world data using mHealth apps in rhinitis, rhinosinusitis and their multimorbidities.
Sousa-Pinto B, Anto A, Berger M, Dramburg S, Pfaar O, Klimek L, Jutel M, Czarlewski W, Bedbrook A, Valiulis A, Agache I, Amaral R, Ansotegui IJ, Bastl K, Berger U, Bergmann KC, Bosnic-Anticevich S, Braido F, Brussino L, Cardona V, Casale T, Canonica GW, Cecchi L, Charpin D, Chivato T, Chu DK, Cingi C, Costa EM, Cruz AA, Devillier P, Durham SR, Ebisawa M, Fiocchi A, Fokkens WJ, Gemicioğlu B, Gotua M, Guzmán MA, Haahtela T, Ivancevich JC, Kuna P, Kaidashev I, Khaitov M, Kvedariene V, Larenas-Linnemann DE, Lipworth B, Laune D, Matricardi PM, Morais-Almeida M, Mullol J, Naclerio R, Neffen H, Nekam K, Niedoszytko M, Okamoto Y, Papadopoulos NG, Park HS, Passalacqua G, Patella V, Pelosi S, Pham-Thi N, Popov TA, Regateiro FS, Reitsma S, Rodriguez-Gonzales M, Rosario N, Rouadi PW, Samolinski B, Sá-Sousa A, Sastre J, Sheikh A, Ulrik CS, Taborda-Barata L, Todo-Bom A, Tomazic PV, Toppila-Salmi S, Tripodi S, Tsiligianni I, Valovirta E, Ventura MT, Valero AA, Vieira RJ, Wallace D, Waserman S, Williams S, Yorgancioglu A, Zhang L, Zidarn M, Zuberbier J, Olze H, Antó JM, Zuberbier T, Fonseca JA, Bousquet J.Clin Transl Allergy. 2022 Nov;12(11):e12208. doi: 10.1002/clt2.12208.PMID: 36434742 Free PMC article.
KMEL References
References
-
- Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315(16):1715‐1725. - PubMed
-
- Pfaar O, Agache I, de Blay F, et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. 2019;74(Suppl 108):3‐25. - PubMed
-
- Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy [see comments]. N Engl J Med. 1999;341(7):468‐475. - PubMed
-
- Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment‐resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117(2):319‐325. - PubMed
-
- Muraro A, Roberts G, Halken S, et al. EAACI guidelines on allergen immunotherapy: executive statement. Allergy. 2018;73(4):739‐743. - PubMed
-
- Bousquet J, Pfaar O, Togias A, et al. 2019 ARIA care pathways for allergen immunotherapy. Allergy. 2019;74(11):2087‐2102. - PubMed
-
- Bousquet J, Schünemann HJ, Togias A, et al. Next‐generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real‐world evidence. J Allergy Clin Immunol. 2020;145(1):70‐80.e3. - PubMed
-
- Brozek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines‐2016 revision. J Allergy Clin Immunol. 2017;140(4):950‐958. - PubMed
-
- Brozek JL, Bousquet J, Banea‐Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466‐476. - PubMed
-
- Paoletti G, DiBona D, Chu DK, et al. Allergen immunotherapy: the growing role of observational and randomised trial ‘Real‐World Evidence’. Allergy. 2021. - PubMed
-
- Devillier P, Molimard M, Ansolabehere X, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. 2019;74(7):1317‐1326. - PubMed
-
- Ji X, Hu L, Wang Y, et al. ‘Mobile Health’ for the management of spondyloarthritis and its application in China. Curr Rheumatol Rep. 2019;21(11):61. - PubMed
-
- Bousquet J, Anto JM, Bachert C, et al. ARIA digital anamorphosis: digital transformation of health and care in airway diseases from research to practice. Allergy. 2021;76(1):168‐190. - PubMed
-
- Bedard A, Anto JM, Fonseca JA, et al. Correlation between work impairment, scores of rhinitis severity and asthma using the MASK‐air(®) App. Allergy. 2020;75(7):1672‐1688. - PubMed
-
- Pfaar O, Sousa‐Pinto B, Devillier P, et al. Effects of allergen immunotherapy in the MASK‐air study: a proof‐of‐concept analysis. Allergy. 2021. - PubMed
-
- Bedard A, Basagana X, Anto JM, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: the MASK study. J Allergy Clin Immunol. 2019;144(1):135‐143.e6. - PubMed
-
- Laune D, Arnavielhe S, Viart F, et al. Adaptation of the General data Protection Regulation (GDPR) to a smartphone app for rhinitis and asthma (MASK‐air®). Rev Mal Respir. 2019;36(9):1019‐1031. - PubMed
-
- Bedard A, Sofiev M, Arnavielhe S, et al. Interactions between air pollution and pollen season for rhinitis using mobile technology: a MASK‐POLLAR study. J Allergy Clin Immunol Pract. 2020;8(3):1063‐1073. - PubMed
-
- Puga JL, Krzywinski M, Altman N. Points of significance. Bayesian networks. Nat Methods. 2015;12(9):799‐800. - PubMed
-
- Bousquet J, Jutel M, Pfaar O, et al. The role of mobile health technologies in stratifying patients for AIT and its cessation. The ARIA‐EAACI perspective. J Allergy Clin Immunol Pract. 2021. - PubMed
-
- Pfaar O, Angier E, Muraro A, Halken S, Roberts G. Algorithms in allergen immunotherapy in allergic rhinoconjunctivitis. Allergy. 2020;75(9):2411‐2414. - PubMed
-
- Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765‐798. - PubMed
-
- Bousquet J, Devillier P, Anto JM, et al. Daily allergic multimorbidity in rhinitis using mobile technology: a novel concept of the MASK study. Allergy. 2018;73(8):1622‐1631. - PubMed
-
- Amaral R, Bousquet J, Pereira AM, et al. Disentangling the heterogeneity of allergic respiratory diseases by latent class analysis reveals novel phenotypes. Allergy. 2019;74(4):698‐708. - PubMed
-
- Pfaar O, Agache I, Bergmann KC, et al. Placebo effects in allergen immunotherapy ‐ an EAACI task force position paper. Allergy. 2021;76(3):629‐647. - PubMed
-
- Zhu W, Gao P, Zhang Q, Chen J. Efficacy and safety of subcutaneous immunotherapy for local allergic rhinitis: a Meta‐Analysis of Randomized Controlled Trials. Am J Rhinol Allergy. 2021. - PubMed
-
- Bousquet J, Bewick M, Arnavielhe S, et al. Work productivity in rhinitis using cell phones: the MASK pilot study. Allergy. 2017;72(10):1475‐1484. - PubMed