First insights into the phylogenetic diversity of Mycobacterium tuberculosis in Kuwait and evaluation of REBA MTB-MDR assay for rapid detection of MDR-TB
Affiliations
Affiliations
- Department of Microbiology, Faculty of Medicine, Kuwait University, Safat, Kuwait.
- Kuwait National TB Control Laboratory, Shuwaikh, Kuwait.
- Department of Infection and Immunity, Mycobacteriology Research Section, King Faisal Special Hospital and Research Center (KFSH & RC), Riyadh, Saudi Arabia.
Abstract
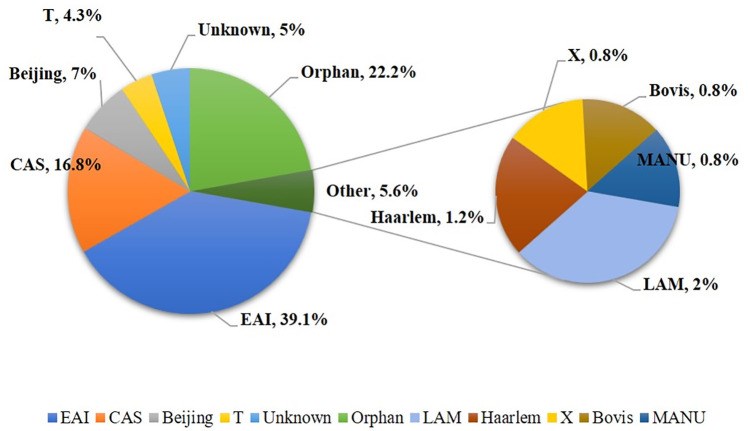
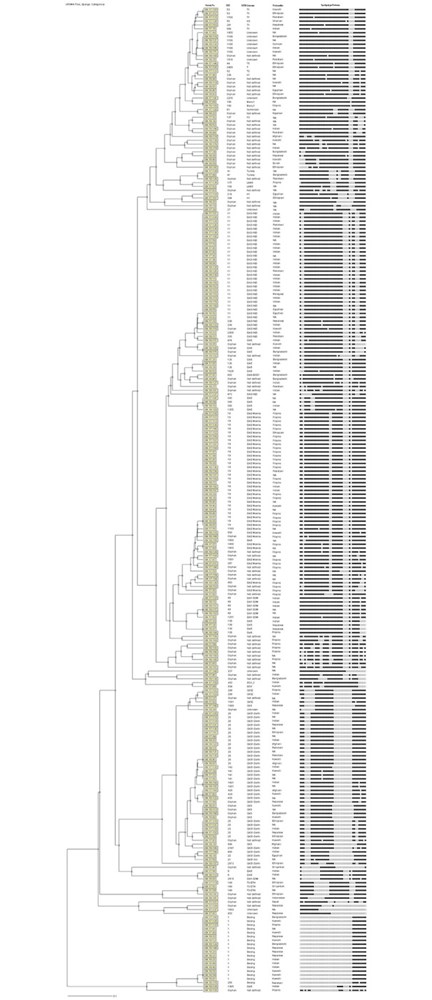
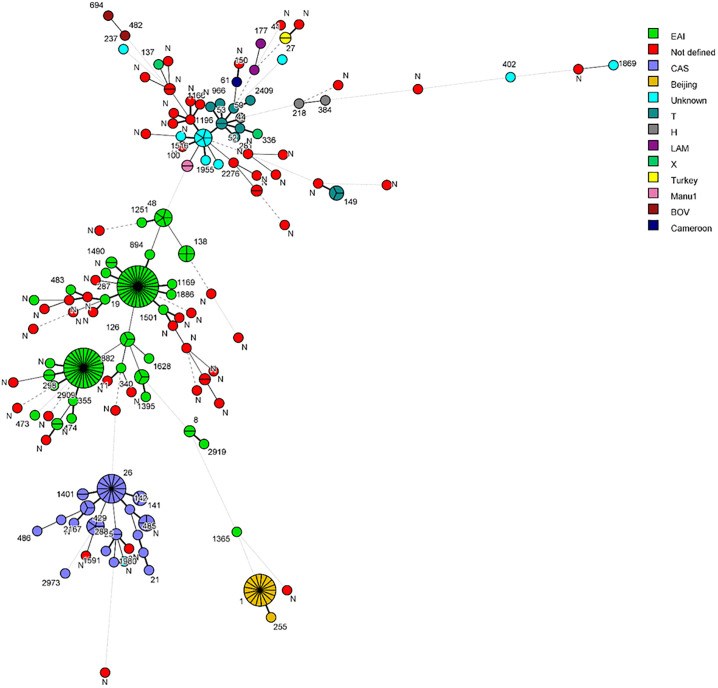
Early detection of Mycobacterium tuberculosis (Mtb) in clinical specimens, its susceptibility to anti-TB drugs and disruption of infection transmission to new hosts are essential components for global tuberculosis (TB) control efforts. This study investigated major Mtb genotypes circulating in Kuwait and evaluated the performance of REBA MTB-MDR (REBA) test in comparison to GenoType MTBDRplus (gMTBDR+) assay for rapid detection of resistance of Mtb to isoniazid and rifampicin (MDR-TB). M. tuberculosis isolates (n = 256) originating predominantly from expatriate patients during a 6-month period were tested by spoligotyping and a dendrogram was created by UPGMA using MIRU-VNTRplus software. Phenotypic drug susceptibility testing (DST) was performed by MGIT 960 system. Genotypic DST for isoniazid and rifampicin was done by REBA and gMTBDR+ assays. Spoligotyping assigned 188 (73.4%) isolates to specific spoligotype international type (SIT) while 68 isolates exhibited orphan patterns. All major M. tuberculosis lineages were detected and EAI, CAS and Beijing families were predominant. Phylogenetic tree showed 131 patterns with 105 isolates exhibiting a unique pattern while 151 isolates clustered in 26 patterns. Fifteen isolates were resistant to one/more drugs. REBA and gMTBDR+ detected isoniazid resistance in 11/12 and 10/12 and rifampicin resistance in 4/5 and 4/5 resistant isolates, respectively. The diversity of SIT patterns are highly suggestive of infection of most expatriate patients with unique Mtb strains, likely acquired in their native countries before their arrival in Kuwait. Both, REBA and gMTBDR+ assays performed similarly for detection of resistance of Mtb to isoniazid and rifampicin for rapid detection of MDR-TB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
Al-Mutairi NM, Ahmad S, Mokaddas EM.Eur J Med Res. 2019 Dec 5;24(1):38. doi: 10.1186/s40001-019-0397-2.PMID: 31806020 Free PMC article.
Cho E, Shamputa IC, Kwak HK, Lee J, Lee M, Hwang S, Jeon D, Kim CT, Cho S, Via LE, Barry CE 3rd, Lee JS.BMC Infect Dis. 2013 Oct 15;13:478. doi: 10.1186/1471-2334-13-478.PMID: 24128118 Free PMC article.
Al-Mutairi NM, Ahmad S, Mokaddas E.Int J Tuberc Lung Dis. 2011 Jan;15(1):110-5.PMID: 21276306
Eshetie S, Gizachew M, Dagnew M, Kumera G, Woldie H, Ambaw F, Tessema B, Moges F.BMC Infect Dis. 2017 Mar 20;17(1):219. doi: 10.1186/s12879-017-2323-y.PMID: 28320336 Free PMC article. Review.
Li H, Yuan J, Duan S, Pang Y.WIREs Mech Dis. 2022 Nov;14(6):e1573. doi: 10.1002/wsbm.1573. Epub 2022 Jun 26.PMID: 35753313 Review.
KMEL References
References
-
- Hingley-Wilson SM, Sambandamurthy VK, Jacobs WR Jr. Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat Immunol. 2003; 4: 949–955. - PubMed
-
- World Health Organization. Global tuberculosis report 2020. https://www.who.int/tb/publications/global_report/en/. WHO; Geneva, Switzerland, (accessed on January 31, 2022).
-
- Yates TA, Khan PY, Knight GM, Taylor JG, McHugh TD, Lipman M, et al.. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis. 2016; 16: 227–238. - PubMed
-
- Driscoll JR. Spoligotyping for molecular epidemiology of the Mycobacterium tuberculosis complex. Methods Mol Biol. 2009; 551: 117–128. - PubMed
-
- Merker M, Kohl TA, Niemann S, Supply P. The Evolution of Strain Typing in the Mycobacterium tuberculosis Complex. Adv Exp Med Biol. 2017; 1019: 43–78. - PubMed
-
- Jeon S, Lim N, Park S, Park M, Kim S. Comparison of PFGE, IS6110-RFLP, and 24-Locus MIRU-VNTR for Molecular Epidemiologic Typing of Mycobacterium tuberculosis Isolates with Known Epidemic Connections. J Microbiol Biotechnol. 2018; 28: 338–346. - PubMed
-
- Havumaki J, Hillemann D, Ismail N, Omar SV, Georghiou SB, Schumacher SG, et al.. Comparative accuracy of the REBA MTB MDR and Hain MTBDRplus line probe assays for the detection of multidrug-resistant tuberculosis: A multicenter, non-inferiority study. PLoS One. 2017; 12: e0173804. doi: 10.1371/journal.pone.0173804 - DOI - PMC - PubMed
-
- Al-Mutairi NM, Ahmad S, Mokaddas E. Molecular screening versus phenotypic susceptibility testing of multidrug-resistant Mycobacterium tuberculosis isolates for streptomycin and ethambutol. Microb Drug Resist. 2018; 24: 923–931. - PubMed
-
- Mokaddas E, Ahmad S, Samir I. Secular trends in susceptibility patterns of Mycobacterium tuberculosis isolates in Kuwait, 1996–2005. Int J Tuberc Lung Dis. 2008; 12: 319–325. - PubMed
-
- Abal AT, Ahmad S, Mokaddas E. Variations in the occurrence of the S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Kuwait. Microb Drug Resist. 2002; 8: 99–105. - PubMed
-
- Mokaddas E, Ahmad S. Development and evaluation of a multiplex PCR for rapid detection and differentiation of Mycobacterium tuberculosis complex members from non-tuberculous mycobacteria. Jpn J Infect Dis. 2007; 60: 140–144. - PubMed
-
- Demay C, Liens B, Burguiere T, Hill V, Couvin D, Millet J, et al.. SITVITWEB—a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol. 2012; 12: 755–766. - PubMed
-
- Al-Mutairi NM, Ahmad S, Mokaddas E. Performance comparison of four methods for detecting multidrug-resistant Mycobacterium tuberculosis strains. Int J Tuberc Lung Dis. 2011; 15: 110–115. - PubMed
-
- Merza MA, Farnia P, Salih AM, Masjedi MR, Velayati AA. The most predominant spoligopatterns of Mycobacterium tuberculosis isolates among Iranian, Afghan-immigrant, Pakistani and Turkish tuberculosis patients: a comparative analysis. Chemotherapy. 2010; 56: 248–257. - PubMed
-
- Hoffner S, Sahebi L, Ansarin K, Sabour S, Mohajeri P. Mycobacterium tuberculosis of the Beijing genotype in Iran and the World Health Organization Eastern Mediterranean Region: A meta-analysis. Microb Drug Resist. 2018; 24: 693–698. - PubMed
-
- Poonawala H, Kumar N, Peacock SJ. A review of published spoligotype data indicates the diversity of Mycobacterium tuberculosis from India is under-represented in global databases. Infect Genet Evol. 2020; 78: 104072. - PubMed
-
- Shah Y, Poudel A, Maharjan B, Thapa J, Yamaguchi T, Diab HM, et al.. Genetic diversity of Mycobacterium tuberculosis Central Asian Strain isolates from Nepal and comparison with neighboring countries. Trans R Soc Trop Med Hyg. 2019; 113: 203–211. - PubMed
-
- Uddin MKM, Ahmed M, Islam MR, Rahman A, Khatun R, Hossain MA, et al.. Molecular characterization and drug susceptibility profile of Mycobacterium tuberculosis isolates from Northeast Bangladesh. Infect Genet Evol. 2018; 65: 136–143. - PubMed
-
- Couvin D, Reynaud Y, Rastogi N. Two tales: Worldwide distribution of Central Asian (CAS) versus ancestral East-African Indian (EAI) lineages of Mycobacterium tuberculosis underlines a remarkable cleavage for phylogeographical, epidemiological and demographical characteristics. PLoS One. 2019; 14: e0219706. doi: 10.1371/journal.pone.0219706 - DOI - PMC - PubMed
-
- Couvin D, David A, Zozio T, Rastogi N. Macro-geographical specificities of the prevailing tuberculosis epidemic as seen through SITVIT2, an updated version of the Mycobacterium tuberculosis genotyping database. Infect Genet Evol. 2019; 72: 31–43. - PubMed
-
- Montoya JC, Murase Y, Ang C, Solon J, Ohkado A. A molecular epidemiologic analysis of Mycobacterium tuberculosis among Filipino patients in a suburban community in the Philippines. Kekkaku. 2013; 88: 543–52. - PubMed
-
- Joseph BV, Soman S, Radhakrishnan I, Hill V, Dhanasooraj D, Kumar RA, et al.. Molecular epidemiology of Mycobacterium tuberculosis isolates from Kerala, India using IS6110-RFLP, spoligotyping and MIRU-VNTRs. Infect Genet Evol. 2013; 16: 157–164. - PubMed
-
- Merker M, Blin C, Mona S, Duforet-Frebourg N, Lecher S, Willery E, et al.. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015; 47: 242–249. - PubMed
-
- Al-Maniri A, Singh JP, Al-Rawas O, Al Busaidi S, Al Balushi L, Ahmed I, et al.. A snapshot of the biodiversity and clustering of Mycobacterium tuberculosis in Oman using spoligotyping. Int J Tuberc Lung Dis. 2010; 14: 994–1000. - PubMed
-
- Al-Hajoj S, Varghese B, Al-Habobe F, Shoukri MM, Mulder A, van Soolingen D. Current trends of Mycobacterium tuberculosis molecular epidemiology in Saudi Arabia and associated demographical factors. Infect Genet Evol. 2013; 16: 362–368. - PubMed
-
- Ahmed MM, Mohammed SH, Nasurallah HA, Ali MM, Couvin D, Rastogi N. Snapshot of the genetic diversity of Mycobacterium tuberculosis isolates in Iraq. Int J Mycobacteriol. 2014; 3: 184–196. - PubMed
-
- Kiepiela P, Bishop KS, Smith AN, Roux L, York DF. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber Lung Dis. 2000; 80: 47–56. - PubMed