Multicentre Observational Study of Treatment Satisfaction with Cladribine Tablets in the Management of Relapsing Multiple Sclerosis in the Arabian Gulf: The CLUE Study
Affiliations
Affiliations
- Rashid Hospital and Dubai Medical College and Dubai Health Authority (DHA), P.O. Box 4545, Dubai, UAE. jsInshasi@emirates.net.ae.
- Ibn Sina Hospital, Kuwait, Kuwait.
- Faculty of Medicine, Minia University, Minia, Egypt.
- Sheikh Shakhbout Medical City, Abu Dhabi, UAE.
- Tawam Hospital, Abu Dhabi, UAE.
- College of Medicine and Health Science, United Arab Emirates University, Abu Dhabi, UAE.
- Al Zahra Hospital, Dubai, UAE.
- Mediclinic City Hospital, Dubai, UAE.
- Rashid Hospital and Dubai Medical College and Dubai Health Authority (DHA), P.O. Box 4545, Dubai, UAE.
- American Center for Psychiatry and Neurology, Dubai, UAE.
- Cleveland Clinic Abu Dhabi, Abu Dhabi, UAE.
- Merck Serono Middle East FZ Ltd, Dubai, UAE.
- Amiri Hospital, Sharq, Kuwait.
Abstract
Introduction: Inconvenient administration and side effects of some disease-modifying therapies (DMTs) for relapsing multiple sclerosis (RMS) can deter adherence. We evaluated treatment satisfaction with cladribine tablets (CladT) for RMS in the Arabian Gulf.
Methods: This was a non-interventional, multicentre, prospective observational study in non-pregnant/lactating adults (aged ≥ 18 years) with RMS eligible for 1st treatment with CladT (EU labelling). The primary outcome was overall treatment satisfaction at 6 months (Treatment Satisfaction Questionnaire for Medication [TSQM]-14, v. 1.4), Global Satisfaction subscale. Secondary endpoints were TSQM-14 scores for convenience, satisfaction with side effects and satisfaction with effectiveness. Patients provided written informed consent.
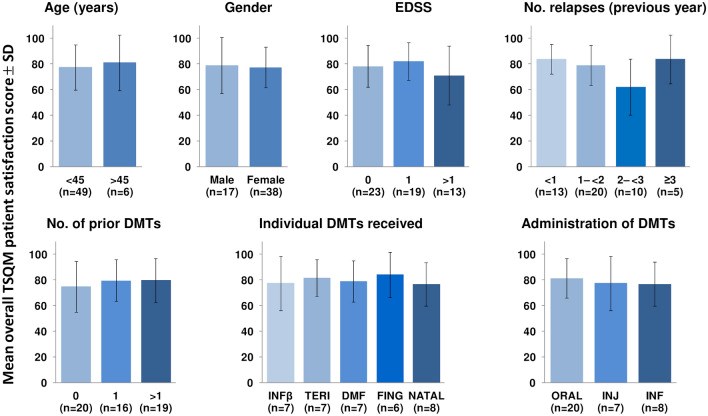
Results: Of 63 patients screened, 58 received CladT and 55 completed the study. Mean age was 33 ± 9 years; mean weight 73 ± 17 kg; 31% male/69% female; mostly from the United Arab Emirates (52%) or Kuwait (30%). All had RMS (mean 0.9 ± 1.1 relapses in the past year), mean Expanded Disability Status Scale (EDSS) 1.4 ± 1.2; 36% were DMT-naïve. Mean [95% CI] score was high for overall treatment satisfaction (77.8 [73.0-82.6]), ease of use (87.4 [83.7-91.0]), tolerability (94.2 [91.0-97.3]) and effectiveness (76.2 [71.6-80.7]). Scores were similar irrespective of DMT history, age, gender, relapse history or EDSS. No relapses or serious treatment-emergent adverse events (TEAE) occurred. Two severe TEAE occurred (fatigue, headache) and 16% reported lymphopenia (two cases of grade 3 lymphopenia). Absolute lymphocyte counts at baseline and 6 months were 2.2 ± 0.8 × 109/L and 1.3 ± 0.3 × 109/L, respectively.
Conclusions: Treatment satisfaction, ease of use, tolerability and patient-perceived effectiveness for CladT were high, irrespective of baseline demographics, disease characteristics and prior treatment.
Keywords: Cladribine tablets; Disease-modifying therapy; Multiple sclerosis; Patient-reported outcomes.
Conflict of interest statement
Ahmed Shatila received honoraria for lectures (Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Boehringeringer Ingelheim, Biologix) and for advisory boards (Sanofi-Genzyme, Roche, Novartis, Pfizer, Biologix); educational conferences travel and registration and hotel accommodation has been sponsored by Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Biologix. Raed Alroughani received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Taoufik Alsaadi Alsaadi received consulting fees, honoraria, and research grants from Novartis, GlaxoSmithKline, Merck, Pfizer, and Hekma. Jihad Inshasi and Samar Farouk received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. Victoria Mifsud received honoraria for participation in Advisory boards from Merck, Roche and Novartis. Amir Boshra and Shatha Sayegh are employees of Merck Serono Middle East FZ-Ltd. Mona Thakre, Abubaker Almadani, Beatrice Benedetti, Deeb Kayed, Derk Krieger, Miklos Szolics, Anu Jacob and Ali Hassan have nothing to disclose beyond the current work.
Figures
Similar articles
Brochet B, Hupperts R, Langdon D, Solari A, Piehl F, Lechner-Scott J, Montalban X, Selmaj K, Valis M, Rejdak K, Havrdova EK, Patti F, Alexandri N, Nolting A, Keller B.Mult Scler Relat Disord. 2022 Jan;57:103385. doi: 10.1016/j.msard.2021.103385. Epub 2021 Nov 9.PMID: 35158476 Clinical Trial.
Turčáni P, Mašková J, Húska J.Patient Prefer Adherence. 2020 Jul 7;14:1129-1135. doi: 10.2147/PPA.S254427. eCollection 2020.PMID: 32753853 Free PMC article.
Oreja-Guevara C, Brownlee W, Celius EG, Centonze D, Giovannoni G, Hodgkinson S, Kleinschnitz C, Havrdova EK, Magyari M, Selchen D, Vermersch P, Wiendl H, Van Wijmeersch B, Salloukh H, Yamout B.Mult Scler Relat Disord. 2023 Jan;69:104459. doi: 10.1016/j.msard.2022.104459. Epub 2022 Dec 8.PMID: 36565573 Review.
Berkovich R, Negroski D, Wynn D, Sellers D, Bzdek KG, Lublin AL, Rawlings AM, Quach C, Wells DP, Dumlao M, Bora A, Ranno AE, Luo KL, Chavin J, Hua LH, Becker D.Mult Scler Relat Disord. 2023 Feb;70:104472. doi: 10.1016/j.msard.2022.104472. Epub 2022 Dec 18.PMID: 36566698
Mitoxantrone: a review of its use in multiple sclerosis.
Scott LJ, Figgitt DP.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010.PMID: 15089110 Review.
KMEL References
References
-
- Practice guideline recommendations summary Disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019;92:112. doi: 10.1212/WNL.0000000000006722. - DOI - PubMed
-
- Brochet B, Hupperts R, Langdon D, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord. 2022;57:103385. doi: 10.1016/j.msard.2021.103385. - DOI - PubMed
-
- Räuber S, Pawlitzki M, Korsen M, et al. A national, multi-center study in Germany to assess implementation of infusion management, treatment satisfaction and quality of life in MS patients receiving alemtuzumab. Mult Scler Relat Disord. 2022;59:103670. doi: 10.1016/j.msard.2022.103670. - DOI - PubMed
-
- Cook S, Comi C, Giovannoni G, et al. Rates of lymphopenia in years 1–4 in patients with relapsing multiple sclerosis treated annually with cladribine tablets. Abstract 039 at the Australian & New Zealand Association of Neurologists (ANZAN) 2018 meeting, May 29th–June 1st, Darwin, Australia. J Neurol Neurosurg Psychiatr 2018;89Abstract 039.
-
- Nasir M, Rabvukwa P, Fuller S, Chaudhuri A, Mattoscio M. Real world data evaluating efficacy and safety of oral cladribine for multiple sclerosis. J Neurol Neurosurg Psychiatr. 2022;93:e2. doi: 10.1136/jnnp-2022-abn2.169. - DOI
-
- Multiple Sclerosis International Federation. Atlas of MS 3rd edition (2020). https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidem.... Accessed Feb 2023.
-
- Multiple Sclerosis Trust. Expanded Disability Status Scale (EDSS). https://www.mstrust.org.uk/a-z/expanded-disability-status-scale-edss. Accessed Feb 2023.