Mitochondrial DNA copy number in autism spectrum disorder and attention deficit hyperactivity disorder: a systematic review and meta-analysis
Affiliations
Affiliations
- Department of Molecular Medicine and Al-Jawhara Centre for Molecular Medicine, Genetics, and Inherited Disorders, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain.
- Department of Psychiatry, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain.
- Government Hospital, Manama, Bahrain.
- Department of Medical Laboratory, Faculty of Allied Health, Kuwait University, Kuwait City, Kuwait.
- Faculty of Medicine, Dar Al Uloom University, Riyadh, Saudi Arabia.
- Department of Psychiatry, Northern Ontarion School of Medicine University, Thunder Bay, ON, Canada.
Abstract
Background: Several reports suggest that altered mitochondrial DNA copy number (mtDNA-cn), a common biomarker for aberrant mitochondrial function, is implicated in autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), but the results are still elusive.
Methods: A meta-analysis was performed to summarize the current indication and to provide a more precise assessment of the mtDNA-cn in ASD and ADHD. A search in the MEDLINE-PubMed, Scopus, and EMBASE databases was done to identify related studies up to the end of February 2023. The meta-analysis was conducted according to recommendations of the Cochrane Handbook of Systematic Reviews.
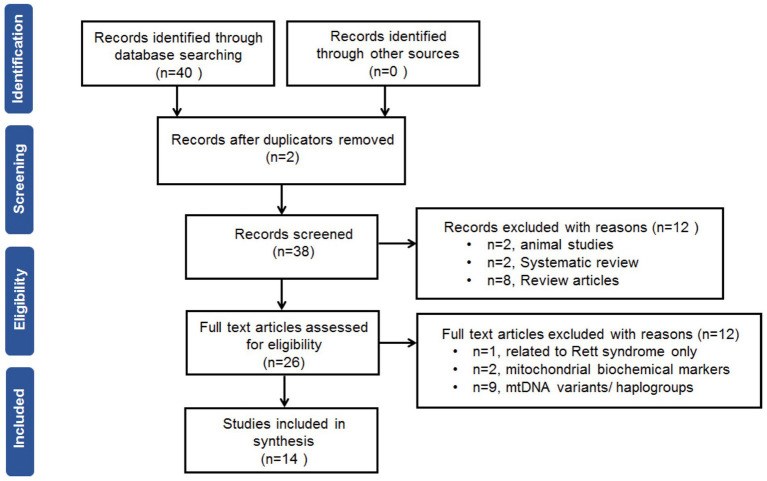
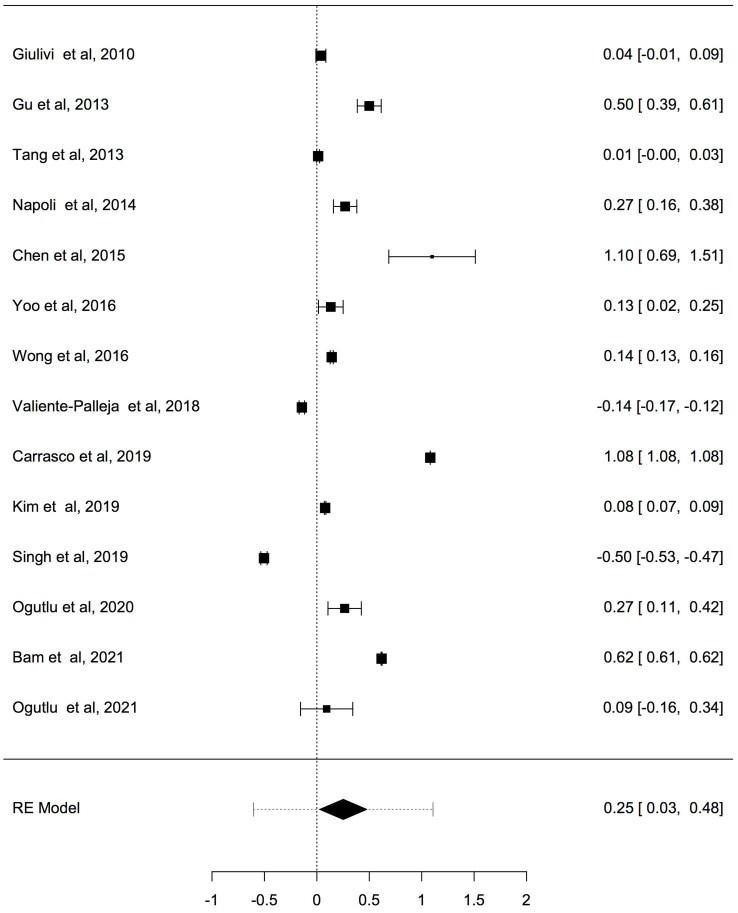
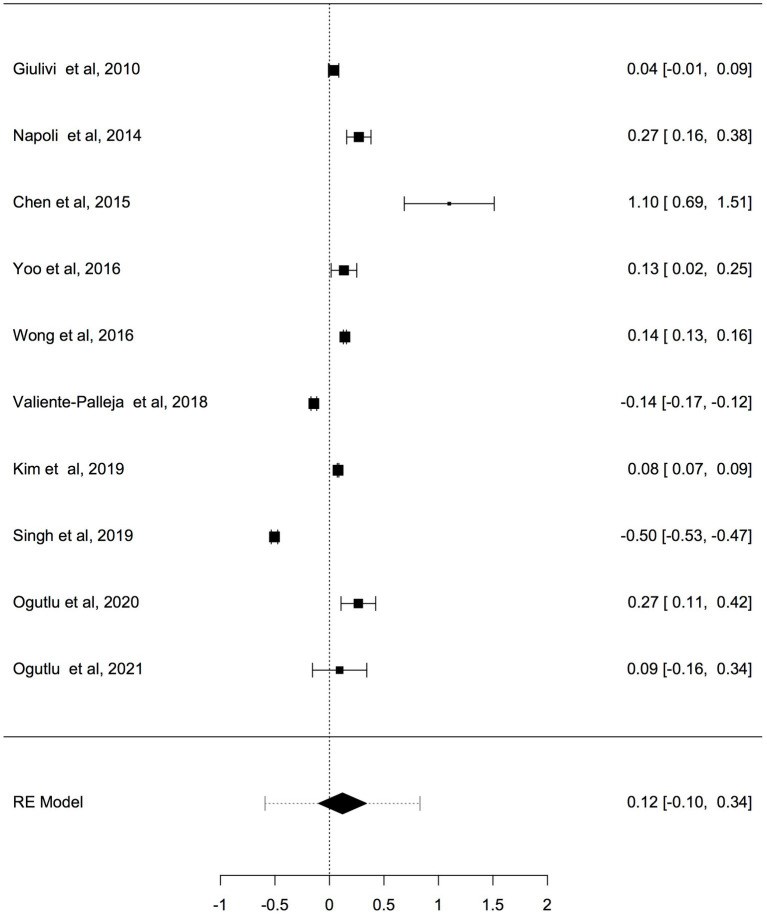
Results: Fourteen studies involving 666 cases with ASD and ADHD and 585 controls were collected and judged relevant for the systematic review and meta-analysis. The pooled results by a random effects meta-analysis was reported as a geometric mean of the estimated average response ratio and 95% confidence interval. Overall analysis of studies reported differences in mtDNA-cn in blood samples (k = 10) and non-blood samples (brain tissues and oral samples; k = 4) suggested significantly higher mtDNA-cn in patients compared to controls (p = 0.0275). Sub-analysis by stratifying studies based on tissue type, showed no significant increase in mtDNA-cn in blood samples among patients and controls (p = 0.284). Conversely, higher mtDNA-cn was observed in non-blood samples in patients than in controls (p = 0.0122). Further stratified analysis based on blood-cell compositions as potential confounds showed no significant difference in mtDNA-cn in peripheral blood samples of patients comparted to controls (p = 0.074). In addition, stratified analysis of aged-matched ASD and ADHD patients and controls revealed no significant difference in mtDNA-cn in blood samples between patients and controls (p = 0.214), whereas a significant increase in mtDNA-cn was observed in non-blood samples between patients and controls (p < 0.001). Finally, when the mtDNA-cn was analyzed in blood samples of aged-matched patients with ASD (peripheral blood, leukocytes, and PBMCs) or ADHD (peripheral blood), no significant difference in mtDNA-cn was observed between ASD patients and controls (p = 0.385), while a significant increase in mtDNA-cn was found between ADHD patients and controls (p = 0.033).
Conclusion: In this first meta-analysis of the evaluation of mtDNA-cn in ASD/ADHD, our results show elevated mtDNA-cn in ASD and ADHD, further emphasizing the implication of mitochondrial dysfunction in neurodevelopmental disorders. However, our results indicate that the mtDNA-cn in blood is not reflected in other tissues in ASD/ADHD, and the true relationship between blood-derived mtDNA-cn and ASD/ADHD remains to be defined in future studies. The importance of blood-cell compositions as confounders of blood-based mtDNA-cn measurement and the advantages of salivary mtDNA-cn should be considered in future studies. Moreover, the potential of mtDNA-cn as a biomarker for mitochondrial malfunction in neurodevelopmental disorders deserves further investigations.
Keywords: ADHD; ASD; mitochondrial dysfunction; mtDNA copy number; neurodevelopmental disorders.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
KMEL References
References
-
- American Psychiatric Association . DSM-V. St. Louis: American Psychiatric Association; (2013).
-
- van der Meer JMJ, Oerlemans AM, van Steijn DJ, Lappenschaar MGA, de Sonneville LMJ, Buitelaar JK, et al. . Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry. (2012) 51:1160–1172.e3. doi: 10.1016/j.jaac.2012.08.024, PMID: - DOI - PubMed
-
- Mulligan A, Anney RJL, O’Regan M, Chen W, Butler L, Fitzgerald M, et al. . Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord. (2009) 39:197–209. doi: 10.1007/s10803-008-0621-3, PMID: - DOI - PubMed
-
- Page MJ, Higgins JP, Sterne JA. Assessing risk of bias due to missing results in a synthesis. (2019) Cochrane handbook for systematic reviews of interventions Higgins J. P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., et al. . Wiley, Hoboken, NJ.
-
- Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. JEBS. (2005) 30:261–93. doi: 10.3102/10769986030003261 - DOI
-
- Cochran WG. Some methods for strengthening the common χ 2 tests. Biometrics. (1954) 10:417–51. doi: 10.2307/3001616 - DOI
-
- Gu F, Chauhan V, Kaur K, Brown WT, LaFauci G, Wegiel J, et al. . Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl Psychiatry. (2013) 3:e299–9. doi: 10.1038/tp.2013.68, PMID: - DOI - PMC - PubMed
-
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. . Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. (2003) 33:427–33. doi: 10.1023/A:1025014929212 - DOI - PubMed
-
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. . Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36:980–8. doi: 10.1097/00004583-199707000-00021, PMID: - DOI - PubMed
-
- Yang SY, Castellani CA, Longchamps RJ, Pillalamarri VK, O’Rourke B, Guallar E, et al. . Blood-derived mitochondrial DNA copy number is associated with gene expression across multiple tissues and is predictive for incident neurodegenerative disease. Genome Res. (2021) 31:349–58. doi: 10.1101/gr.269381.120, PMID: - DOI - PMC - PubMed
-
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernstrom J, et al. . Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology. (2018) 43:1557–64. doi: 10.1038/s41386-017-0001-9, PMID: - DOI - PMC - PubMed
-
- Ridout KK, Parade SH, Kao HT, Magnan S, Seifer R, Porton B, et al. . Childhood maltreatment, behavioral adjustment, and molecular markers of cellular aging in preschool-aged children: a cohort study. Psychoneuroendocrinology. (2019) 107:261–9. doi: 10.1016/j.psyneuen.2019.05.015, PMID: - DOI - PMC - PubMed