COVID-19 vaccination in people with multiple sclerosis, real-life experience
Affiliations
Affiliations
- Division of Neurology, Amiri Hospital, Arabian Gulf Street, Sharq 13041, Kuwait; MS Clinic, Ibn Sina Hospital, P.O. Box 25427, Safat 13115, Kuwait. Electronic address: alroughani@gmail.com.
- Department of Neurology, Ibn Sina Hospital, P.O. Box 25427, Safat 13115, Kuwait; Department of Medicine, Faculty of Medicine, Kuwait University, P.O. Box 24923, Safat 13110, Kuwait. Electronic address: jasemkumsa@hotmail.com.
- Department of Neurology, Ibn Sina Hospital, P.O. Box 25427, Safat 13115, Kuwait. Electronic address: dr_fathi2010@yahoo.com.
- Department of Medicine, Faculty of Medicine, Kuwait University, P.O. Box 24923, Safat 13110, Kuwait. Electronic address: malak.almojel@gmail.com.
- Department of Neurology, Ibn Sina Hospital, P.O. Box 25427, Safat 13115, Kuwait; Department of Neurology and Psychiatry, Minia University, P.O. Box 61519, Minia 61111, Egypt. Electronic address: samerelshayb@hotmail.com.
Abstract
Background: Vaccination against the severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2) virus is recommended in multiple sclerosis (MS) to reduce the risk of complications from Coronavirus disease 2019 (COVID-19) infection. These vaccines were not investigated in people with MS (PWMS).
Objective: This study aimed to report the short-term safety of the COVID-19 vaccines among PWMS.
Methods: Pfizer-BioNTech mRNA (BNT162b2) vaccine and Oxford-Astra Zenecaa chimpanzee adenovirus-vectored (ChAdOx1 nCoV-19) vaccine have been approved to be used in Kuwait since December 2021. PWMS registered in Kuwait national registry were contacted by phone, WhatsApp, or through face-to-face interviews and were invited to complete our questionnaire. Demographic, clinical data, symptoms following the vaccine, worsening of pre-existing MS symptoms, and occurrence of relapse were recorded.
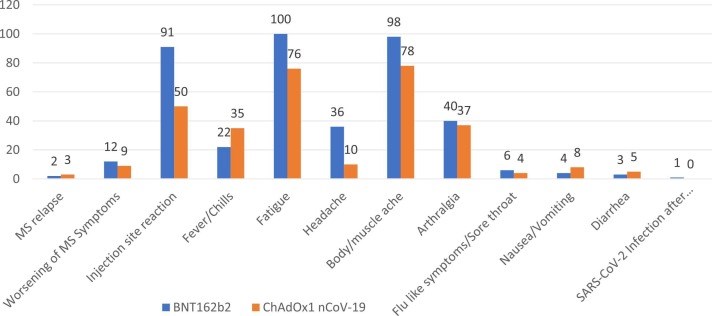
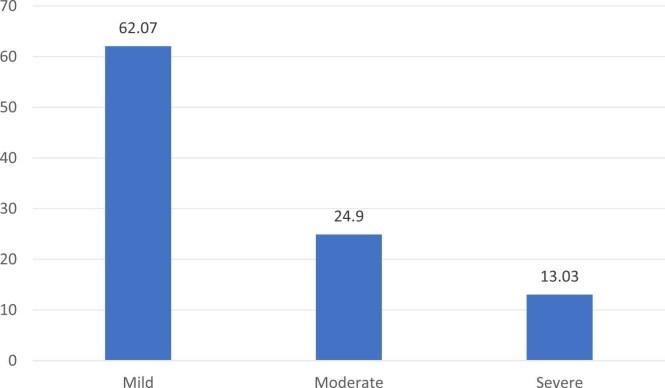
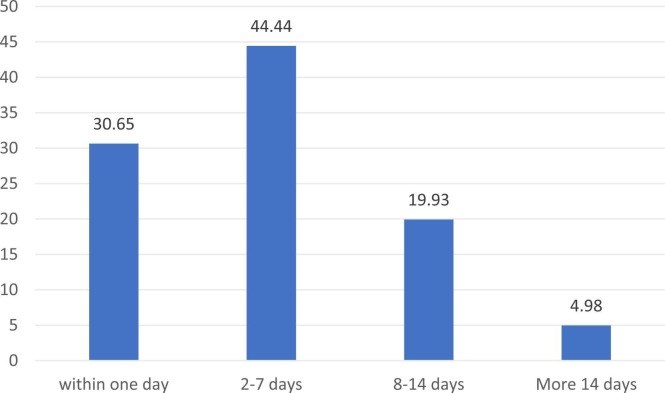
Results: Of the 820 PWMS, 647 completed the questionnaire. Between January 2021 and 31 August 2021, 383 (59.28%) PWMS received at least one dose of the approved vaccinations versus 63.4% of the general population on the same date. Their mean age was 36.82 + 8.80, and most of them, 247 (64.3%), were females. A total of 356 vaccinated cohorts (92.6%) were treated with disease-modifying therapies. Adverse events were reported by 261 (68.15%) subjects. One case of COVID-19 infection was encountered after the first dose of the BNT162b2 vaccine. Twenty-one (5.48%) cases reported worsening of pre-existing MS symptoms after the vaccine. Five patients (1.31%) reported relapse after the COVID-19 vaccine. The most common adverse events of the COVID-19 vaccine were pain at the injection site, fatigue, low-grade fever, and body ache; and resolved within one week. There was no significant association between use of disease modifying therapy (DMT) and COVID-19 vaccine adverse events.
Conclusion: BNT162b2 and ChAdOx1 nCoV-19 are safe for PWMS. No increased risk of relapse activity or worsening of pre-existing MS symptoms.
Keywords: COVID-19; Kuwait; Multiple sclerosis; Vaccination.
Conflict of interest statement
Declaration of Competing Interest RA is an Advisory Board member of Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi- Genzyme, and received honoraria for speaking or consultation fees from Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi-Genzyme. He is also the principal investigator in clinical trials for Biogen, Merck Serono, Novartis, Roche, Sanofi-Genzyme. SFA acted as Advisory Board members of Bayer, Merck Serono, Novartis, Sanofi- Genzyme, and received honoraria for speaking or consultation fees from, Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi-Genzyme. She is also the co-investigator in clinical trials for Biogen, Merck Serono, Novartis, Roche, Sanofi-Genzyme. JA is an Advisory Board member and received honoraria for speaking from Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi- Genzyme. FA and MA declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
COVID-19 Vaccination Reactogenicity in Persons With Multiple Sclerosis.
Briggs FBS, Mateen FJ, Schmidt H, Currie KM, Siefers HM, Crouthamel S, Bebo BF, Fiol J, Racke MK, O'Connor KC, Kolaczkowski LG, Klein P, Loud S, McBurney RN.Neurol Neuroimmunol Neuroinflamm. 2021 Nov 9;9(1):e1104. doi: 10.1212/NXI.0000000000001104. Print 2022 Jan.PMID: 34753828 Free PMC article.
Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC.BMJ. 2021 Aug 26;374:n1931. doi: 10.1136/bmj.n1931.PMID: 34446426 Free PMC article.
Did it hurt? COVID-19 vaccination experience in people with multiple sclerosis.
Allen-Philbey K, Stennett A, Begum T, Johnson AC, MacDougall A, Green S, Dobson R, Giovannoni G, Gnanapavan S, Marta M, Smets I, Turner BP, Baker D, Mathews J, Schmierer K.Mult Scler Relat Disord. 2022 Sep;65:104022. doi: 10.1016/j.msard.2022.104022. Epub 2022 Jul 3.PMID: 35816953 Free PMC article.
Jarius S, Bieber N, Haas J, Wildemann B.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23.PMID: 35737110 Free PMC article. Review.
Anti-SARS-CoV-2 vaccination in people with multiple sclerosis: Lessons learnt a year in.
Pugliatti M, Hartung HP, Oreja-Guevara C, Pozzilli C, Airas L, Alkhawajah M, Grigoriadis N, Magyari M, Van Wijmeersch B, Zakaria M, Linker R, Chan A, Vermersch P, Berger T.Front Immunol. 2022 Oct 17;13:1045101. doi: 10.3389/fimmu.2022.1045101. eCollection 2022.PMID: 36325318 Free PMC article. Review.
Cited by
Frasca L, Ocone G, Palazzo R.Pathogens. 2023 Feb 2;12(2):233. doi: 10.3390/pathogens12020233.PMID: 36839505 Free PMC article. Review.
Safety of COVID-19 vaccines in multiple sclerosis: A systematic review and meta-analysis.
Stefanou MI, Palaiodimou L, Theodorou A, Christodoulou MV, Tzartos JS, Tzanetakos D, Kitsos D, Chondrogianni M, Zouvelou V, Dardiotis E, Tzavellas E, Syrigou E, Benetou V, Paraskevas GP, Tsiodras S, Tsivgoulis G, Giannopoulos S.Mult Scler. 2023 Apr;29(4-5):585-594. doi: 10.1177/13524585221150881. Epub 2023 Feb 1.PMID: 36722184 Free PMC article.
KMEL References
References
-
- World Health Organization, Novel coronavirus situation report: 99. 28 April 2020. 〈https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... pdf?sfvrsn=119fc381_2〉. Accessed 28 Apr 2020.
-
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. - PMC - PubMed
-
- AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis [WWW Document]. URL. 〈https://www.astrazeneca.com/media-ce ntre/press releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html〉. accessed 3.22.21.
-
- M. Kuwait, Kuwait COVID-19 Vaccine, 2021; Available from: 〈https://ourworldindata.org/covid-vaccinations?country=OWID_WRL〉.
-
- Voysey M., Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. - PMC - PubMed
-
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. - PMC - PubMed
-
- COVID-19 Vaccine Guidance for People Living with MS. 〈www.nationalmssociety.org/coronavirus-covid〉- 19-information/multiple-sclerosis-and-coronavirus/ covid-19-vaccine-guidance (accessed 18 January 2021).
-
- COVID-19 vaccination guidance for people with MS 2021 Available from: 〈https://www.msaustralia.org.au/about-ms/covid-19-and-ms/〉 covid-19-vaccination-guidance-people-ms.
-
- COVID-19 mRNA vaccines (Pfizer-BioNTech and Moderna) and MS 2021 Available from: 〈https://mssociety.org.il/en/covid〉 -19-mrna-vaccines-pfizer-biontech-and-moderna-and-ms/.
-
- MS, the coronavirus and vaccines – updated global advice 2021 Available from: 〈https://www.msif.org/news/2020/02/10/the〉- coronavirus-and-ms-what-you-need-to-know/.
-
- MS Treatment Guidelines During the Coronavirus pandemic | National MS Society | National Multiple Sclerosis Society [WWW Document]. URL. 〈https://www.nationalmssociety.org/coronavirus-covid-19-information/multi... avirus/ms-treatment-guidelines-during-coronavirus. accessed 3.21.21.
-
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 - PMC - PubMed
-
- Di Filippo M., Cordioli C., Malucchi S., Annovazzi P., Cavalla P., Clerici V.T., Ragonese P., Nociti V., Radaelli M., Laroni A., Buttari F. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol., Neurosurg. Psychiatry. 2021 - PubMed
-
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. - PubMed
-
- Food, U., et al., Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials, 2007.
-
- Covidvax.live : Live COVID-19 Vaccination Tracker - See vaccinations in real time! [Internet]. Covidvax.live. 2021 [cited 2021 Jun 29]. Available from: 〈https://covidvax.live/location/kwt〉.
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., Belij-Rammerstorfer S. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. - PMC - PubMed
-
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. - DOI - PMC - PubMed
-
- CDC, Possible Side Effects After Getting a COVID-19 Vaccine. Available online at: 〈https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after〉. html (accessed June 15, 2021).
-
- Reyes S., Ramsay M., Ladhani S., Amirthalingam G., Singh N., Cores C., Lambourne J., Marta M., Turner B., Gnanapavan S., Dobson R. Protecting people with multiple sclerosis through vaccination. Pract. Neurol. 2020;20(6):435–445. - PubMed
-
- Nakayama T. Causal relationship between immunological re- sponses and adverse reactions following vaccination. Vaccine. 2019;37(2):366–371. - PubMed
-
- Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a sys- tematic review. J. Neurol. 2017;264(6):1035–1050. - PubMed
-
- Farez M.F., Correale J. Immunizations and risk of multi- ple sclerosis: systematic review and meta-analysis. J. Neurol. 2011;258(7):1197–1206. - PubMed
-
- Web Page: YouGov, 2020, How many Britons are willing to take a coronavirus vaccine? Access date: 3 February 2021. 〈https://yougov.co.uk/topics/health/articles-repor〉 ts/2020/11/16/how-many-britons-are-willing-take-coronavirus-vacc.
-
- MacDonald N.E. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. - PubMed