Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead
William H Goodson 3rd 1, Leroy Lowe 2, David O Carpenter 3, Michael Gilbertson 4, Abdul Manaf Ali 5, Adela Lopez de Cerain Salsamendi 6, Ahmed Lasfar 7, Amancio Carnero 8, Amaya Azqueta 6, Amedeo Amedei 9, Amelia K Charles 10, Andrew R Collins 11, Andrew Ward 12, Anna C Salzberg 13, Annamaria Colacci 14, Ann-Karin Olsen 15, Arthur Berg 13, Barry J Barclay 16, Binhua P Zhou 17, Carmen Blanco-Aparicio 18, Carolyn J Baglole 19, Chenfang Dong 17, Chiara Mondello 20, Chia-Wen Hsu 21, Christian C Naus 22, Clement Yedjou 23, Colleen S Curran 24, Dale W Laird 25, Daniel C Koch 26, Danielle J Carlin 27, Dean W Felsher 28, Debasish Roy 29, Dustin G Brown 30, Edward Ratovitski 31, Elizabeth P Ryan 30, Emanuela Corsini 32, Emilio Rojas 33, Eun-Yi Moon 34, Ezio Laconi 35, Fabio Marongiu 35, Fahd Al-Mulla 36, Ferdinando Chiaradonna 37, Firouz Darroudi 38, Francis L Martin 39, Frederik J Van Schooten 40, Gary S Goldberg 41, Gerard Wagemaker 42, Gladys N Nangami 43, Gloria M Calaf 44, Graeme Williams 45, Gregory T Wolf 46, Gudrun Koppen 47, Gunnar Brunborg 15, H Kim Lyerly 48, Harini Krishnan 41, Hasiah Ab Hamid 49, Hemad Yasaei 50, Hideko Sone 51, Hiroshi Kondoh 52, Hosni K Salem 53, Hsue-Yin Hsu 54, Hyun Ho Park 55, Igor Koturbash 56, Isabelle R Miousse 56, A Ivana Scovassi 20, James E Klaunig 57, Jan Vondráček 58, Jayadev Raju 59, Jesse Roman 60, John Pierce Wise Sr 61, Jonathan R Whitfield 62, Jordan Woodrick 63, Joseph A Christopher 64, Josiah Ochieng 43, Juan Fernando Martinez-Leal 65, Judith Weisz 66, Julia Kravchenko 48, Jun Sun 67, Kalan R Prudhomme 68, Kannan Badri Narayanan 55, Karine A Cohen-Solal 69, Kim Moorwood 12, Laetitia Gonzalez 70, Laura Soucek 71, Le Jian 72, Leandro S D'Abronzo 73, Liang-Tzung Lin 74, Lin Li 75, Linda Gulliver 76, Lisa J McCawley 77, Lorenzo Memeo 78, Louis Vermeulen 79, Luc Leyns 70, Luoping Zhang 80, Mahara Valverde 33, Mahin Khatami 81, Maria Fiammetta Romano 82, Marion Chapellier 83, Marc A Williams 84, Mark Wade 85, Masoud H Manjili 86, Matilde E Lleonart 87, Menghang Xia 21, Michael J Gonzalez 88, Michalis V Karamouzis 89, Micheline Kirsch-Volders 70, Monica Vaccari 14, Nancy B Kuemmerle 90, Neetu Singh 91, Nichola Cruickshanks 92, Nicole Kleinstreuer 93, Nik van Larebeke 94, Nuzhat Ahmed 95, Olugbemiga Ogunkua 43, P K Krishnakumar 96, Pankaj Vadgama 97, Paola A Marignani 98, Paramita M Ghosh 73, Patricia Ostrosky-Wegman 33, Patricia A Thompson 99, Paul Dent 92, Petr Heneberg 100, Philippa Darbre 101, Po Sing Leung 75, Pratima Nangia-Makker 102, Qiang Shawn Cheng 103, R Brooks Robey 104, Rabeah Al-Temaimi 105, Rabindra Roy 63, Rafaela Andrade-Vieira 98, Ranjeet K Sinha 106, Rekha Mehta 59, Renza Vento 107, Riccardo Di Fiore 108, Richard Ponce-Cusi 109, Rita Dornetshuber-Fleiss 110, Rita Nahta 111, Robert C Castellino 112, Roberta Palorini 37, Roslida Abd Hamid 49, Sabine A S Langie 47, Sakina E Eltom 43, Samira A Brooks 113, Sandra Ryeom 114, Sandra S Wise 61, Sarah N Bay 115, Shelley A Harris 116, Silvana Papagerakis 46, Simona Romano 82, Sofia Pavanello 117, Staffan Eriksson 118, Stefano Forte 78, Stephanie C Casey 26, Sudjit Luanpitpong 119, Tae-Jin Lee 120, Takemi Otsuki 121, Tao Chen 122, Thierry Massfelder 123, Thomas Sanderson 124, Tiziana Guarnieri 125, Tove Hultman 126, Valérian Dormoy 127, Valerie Odero-Marah 128, Venkata Sabbisetti 129, Veronique Maguer-Satta 84, W Kimryn Rathmell 113, Wilhelm Engström 126, William K Decker 130, William H Bisson 68, Yon Rojanasakul 131, Yunus Luqmani 132, Zhenbang Chen 43, Zhiwei Hu 133
Affiliations
Affiliations
California Pacific Medical Center Research Institute, 2100 Webster Street #401, San Francisco, CA 94115, USA, Getting to Know Cancer, Room 229A, 36 Arthur Street, Truro, Nova Scotia B2N 1X5, Canada, Lancaster Environment Centre, Lancaster University, Bailrigg, Lancaster LA1 4AP, UK, Institute for Health and the Environment, University at Albany, 5 University Pl., Rensselaer, NY 12144, USA, Getting to Know Cancer, Guelph N1G 1E4, Canada, School of Biotechnology, Faculty of Agriculture Biotechnology and Food Sciences, Sultan Zainal Abidin University, Tembila Campus, 22200 Besut, Terengganu, Malaysia, Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Navarra, Pamplona 31008, Spain, Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers, State University of New Jersey, Piscataway, NJ 08854, USA, Instituto de Biomedicina de Sevilla, Consejo Superior de Investigaciones Cientificas. Hospital Universitario Virgen del Rocio, Univ. de Sevilla., Avda Manuel Siurot sn. 41013 Sevilla, Spain, Department of Experimental and Clinical Medicine, University of Firenze, Florence 50134, Italy, School of Biological Sciences, University of Reading, Hopkins Building, Reading, Berkshire RG6 6UB, UK, Department of Nutrition, University of Oslo, Oslo, Norway, Department of Biochemistry and Biology, University of Bath, Claverton Down, Bath BA2 7AY, UK, Department of Public Health Sciences, College of Medicine, Pennsylvania State University, Hershey, PA 17033, USA, Center for Environmental Carcinogenesis and Risk Assessment, Environmental Protection and Health Prevention Agency, 40126 Bologna, Italy, Department of Chemicals and Radiation, Division of Environmental Medicine, Norwegian Institute of Public Health, Oslo N-0403, Norway, Planet Biotechnologies Inc., St Albert, Alberta T8N 5K4, Canada, Department of Molecular and Cellular Biochemistry, University of Kentucky, Lexington, KY 40508, USA, Spanish National Cancer Research Centre, CNI- 2Getting to Know Cancer, Room 229A, 36 Arthur Street, Truro, Nova Scotia B2N 1X5, Canada, Lancaster Environment Centre, Lancaster University, Bailrigg, Lancaster LA1 4AP, UK.
- 3Institute for Health and the Environment, University at Albany, 5 University Pl., Rensselaer, NY 12144, USA.
- 4Getting to Know Cancer, Guelph N1G 1E4, Canada.
- 5School of Biotechnology, Faculty of Agriculture Biotechnology and Food Sciences, Sultan Zainal Abidin University, Tembila Campus, 22200 Besut, Terengganu, Malaysia.
- 6Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Navarra, Pamplona 31008, Spain.
- 7Department of Pharmacology and Toxicology, Ernest Mario School of Pharmacy, Rutgers, State University of New Jersey, Piscataway, NJ 08854, USA.
- 8Instituto de Biomedicina de Sevilla, Consejo Superior de Investigaciones Cientificas. Hospital Universitario Virgen del Rocio, Univ. de Sevilla., Avda Manuel Siurot sn. 41013 Sevilla, Spain.
- 9Department of Experimental and Clinical Medicine, University of Firenze, Florence 50134, Italy.
- 10School of Biological Sciences, University of Reading, Hopkins Building, Reading, Berkshire RG6 6UB, UK.
- 11Department of Nutrition, University of Oslo, Oslo, Norway.
- 12Department of Biochemistry and Biology, University of Bath, Claverton Down, Bath BA2 7AY, UK.
- 13Department of Public Health Sciences, College of Medicine, Pennsylvania State University, Hershey, PA 17033, USA.
- 14Center for Environmental Carcinogenesis and Risk Assessment, Environmental Protection and Health Prevention Agency, 40126 Bologna, Italy.
- 15Department of Chemicals and Radiation, Division of Environmental Medicine, Norwegian Institute of Public Health, Oslo N-0403, Norway.
- 16Planet Biotechnologies Inc., St Albert, Alberta T8N 5K4, Canada.
- 17Department of Molecular and Cellular Biochemistry, University of Kentucky, Lexington, KY 40508, USA.
- 18Spanish National Cancer Research Centre, CNIO, Melchor Fernandez Almagro, 3, 28029 Madrid, Spain.
- 19Department of Medicine, McGill University, Montreal, Quebec H4A 3J1, Canada.
- 20Istituto di Genetica Molecolare, CNR, Via Abbiategrasso 207, 27100 Pavia, Italy.
- 21Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, 9800 Medical Center Drive, Bethesda, MD 20892-3375, USA.
- 22Department of Cellular and Physiological Sciences, Life Sciences Institute, Faculty of Medicine, The University of British Columbia, Vancouver, British Columbia V5Z 1M9, Canada.
- 23Department of Biology, Jackson State University, Jackson, MS 39217, USA.
- 24Department of Molecular and Environmental Toxicology, University of Wisconsin-Madison, Madison, WI 53706, USA.
- 25Department of Anatomy and Cell Biology, University of Western Ontario, London, Ontario N6A 3K7, Canada.
- 26Stanford University Department of Medicine, Division of Oncology, Stanford, CA 94305, USA.
- 27Superfund Research Program, National Institute of Environmental Health Sciences, Research Triangle Park, NC 27560, USA.
- 28Department of Medicine, Oncology and Pathology, Stanford University, Stanford, CA 94305, USA.
- 29Department of Natural Science, The City University of New York at Hostos Campus, Bronx, NY 10451, USA.
- 30Department of Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO 80523-1680, USA.
- 31Department of Head and Neck Surgery/Head and Neck Cancer Research, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
- 32Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, 20133 Milan, Italy.
- 33Department of Genomic Medicine and Environmental Toxicology, Institute for Biomedical Research, National Autonomous University of Mexico, Mexico City 04510, México.
- 34Department of Bioscience and Biotechnology, Sejong University, Seoul 143-747, Korea.
- 35Department of Biomedical Sciences, University of Cagliari, 09124 Cagliari, Italy.
- 36Department of Pathology, Kuwait University, Safat 13110, Kuwait.
- 37Department of Biotechnology and Biosciences, University of Milano-Bicocca, 20126 Milan, Italy, SYSBIO Centre of Systems Biology, Department of Biotechnology and Biosciences, University of Milano-Bicocca, 20126 Milan, Italy.
- 38Human Safety and Environmental Research, Department of Health Sciences, College of North Atlantic, Doha 24449, State of Qatar.
- 39Lancaster Environment Centre, Lancaster University, Bailrigg, Lancaster LA1 4AP, UK.
- 40Department of Toxicology, NUTRIM School for Nutrition, Toxicology and Metabolism, Maastricht University, Maastricht 6200, The Netherlands.
- 41Department of Molecular Biology, School of Osteopathic Medicine, Rowan University, Stratford, NJ 08084, USA.
- 42Hacettepe University, Center for Stem Cell Research and Development, Ankara 06640, Turkey.
- 43Department of Biochemistry and Cancer Biology, Meharry Medical College, Nashville, TN 37208, USA.
- 44Center for Radiological Research, Columbia University Medical Center, New York, NY 10032, USA, Instituto de Alta Investigacion, Universidad de Tarapaca, Arica, Chile.
- 45School of Biological Sciences, University of Reading, Reading, RG6 6UB, UK.
- 46Department of Otolaryngology - Head and Neck Surgery, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
- 47Environmental Risk and Health Unit, Flemish Institute for Technological Research, 2400 Mol, Belgium.
- 48Department of Surgery, Pathology, Immunology, Duke University Medical Center, Durham, NC 27710, USA.
- 49Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, 43400 Universiti Putra Malaysia, Serdang, Selangor, Malaysia.
- 50Department of Life Sciences, College of Health and Life Sciences and the Health and Environment Theme, Institute of Environment, Health and Societies, Brunel University Kingston Lane, Uxbridge, Middlesex UB8 3PH, UK.
- 51National Institute for Environmental Studies, 16-2 Onogawa, Tsukuba, Ibraki 3058506, Japan.
- 52Department of Geriatric Medicine, Kyoto University Hospital 54 Kawaharacho, Shogoin, Sakyo-ku Kyoto, 606-8507, Japan.
- 53Department of Urology, Kasr Al-Ainy School of Medicine, Cairo University, El Manial, Cairo 11559, Egypt.
- 54Department of Life Sciences, Tzu-Chi University, Hualien 970, Taiwan.
- 55School of Biotechnology, Yeungnam University, Gyeongbuk 712-749, South Korea.
- 56Department of Environmental and Occupational Health, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
- 57Department of Environmental Health, Indiana University, School of Public Health, Bloomington, IN 47405, USA.
- 58Department of Cytokinetics, Institute of Biophysics Academy of Sciences of the Czech Republic, Brno, CZ-61265, Czech Republic.
- 59Regulatory Toxicology Research Division, Bureau of Chemical Safety, Food Directorate, Health Canada, Ottawa, Ontario K1A 0K9, Canada.
- 60Department of Medicine, University of Louisville, Louisville, KY 40202, USA, Robley Rex VA Medical Center, Louisville, KY 40202, USA.
- 61Department of Applied Medical Sciences, University of Southern Maine, 96 Falmouth St., Portland, ME 04104, USA.
- 62Mouse Models of Cancer Therapies Group, Vall d'Hebron Institute of Oncology (VHIO), 08035 Barcelona, Spain.
- 63Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington DC 20057, USA.
- 64Cancer Research UK. Cambridge Institute, University of Cambridge, Robinson Way, Cambridge CB2 0RE, UK.
- 65Department of Cell Biology, Pharmamar-SAU, Avda. De los Reyes, 1. 28770-Colmenar Viejo, Madrid, Spain.
- 66Departments of Obstetrics and Gynecology and Pathology, Pennsylvania State University College of Medicine, Hershey PA 17033, USA.
- 67Department of Biochemistry, Rush University, Chicago, IL 60612, USA.
- 68Environmental and Molecular Toxicology, Environmental Health Science Center, Oregon State University, Corvallis, OR 97331, USA.
- 69Department of Medicine/Medical Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ 08903, USA.
- 70Laboratory for Cell Genetics, Vrije Universiteit Brussel, 1050 Brussels, Belgium.
- 71Mouse Models of Cancer Therapies Group, Vall d'Hebron Institute of Oncology (VHIO), 08035 Barcelona, Spain, Catalan Institution for Research and Advanced Studies (ICREA), Barcelona 08010, Spain.
- 72School of Public Health, Curtin University, Bentley, WA 6102, Australia, Department of Urology, University of California Davis, Sacramento, CA 95817, USA.
- 73Department of Urology, University of California Davis, Sacramento, CA 95817, USA.
- 74Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11031, Taiwan.
- 75School of Biomedical Sciences, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR, The People's Republic of China.
- 76Faculty of Medicine, University of Otago, Dunedin 9054, New Zealand.
- 77Department of Biomedical Engineering and Cancer Biology, Vanderbilt University, Nashville, TN 37235, USA.
- 78Department of Experimental Oncology, Mediterranean Institute of Oncology, Via Penninazzo 7, Viagrande (CT) 95029, Italy.
- 79Center for Experimental Molecular Medicine, Academic Medical Center, Meibergdreef 9, Amsterdam 1105 AZ, The Netherlands.
- 80Division of Environmental Health Sciences, School of Public Health, University of California, Berkeley, CA 94720-7360, USA.
- 81Inflammation and Cancer Research, National Cancer Institute (NCI) (Retired), National Institutes of Health, Bethesda, MD 20892, USA.
- 82Department of Molecular Medicine and Medical Biotechnology, Federico II University of Naples, 80131 Naples, Italy.
- 83Centre De Recherche En Cancerologie, De Lyon, Lyon, U1052-UMR5286, France.
- 84United States Army Institute of Public Health, Toxicology Portfolio-Health Effects Research Program, Aberdeen Proving Ground, Edgewood, MD 21010-5403, USA.
- 85Center for Genomic Science of IIT@SEMM, Fondazione Istituto Italiano di Tecnologia, Via Adamello 16, 20139 Milano, Italy.
- 86Department of Microbiology and Immunology, Virginia Commonwealth University, Massey Cancer Center, Richmond, VA 23298, USA.
- 87Institut De Recerca Hospital Vall D'Hebron, Passeig Vall d'Hebron, 119-129, 08035 Barcelona, Spain.
- 88University of Puerto Rico, Medical Sciences Campus, School of Public Health, Nutrition Program, San Juan 00921, Puerto Rico.
- 89Department of Biological Chemistry, Medical School, University of Athens, Institute of Molecular Medicine and Biomedical Research, 10676 Athens, Greece.
- 90Geisel School of Medicine at Dartmouth, Hanover, NH 03755, USA, Geisel School of Medicine at Dartmouth, Hanover, NH 03755, USA.
- 91Advanced Molecular Science Research Centre (Centre for Advanced Research), King George's Medical University, Lucknow, Uttar Pradesh 226 003, India.
- 92Departments of Neurosurgery and Biochemistry and Massey Cancer Center, Virginia Commonwealth University, Richmond, VA 23298, USA.
- 93Integrated Laboratory Systems Inc., in support of the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, RTP, NC 27709, USA.
- 94Analytische, Milieu en Geochemie, Vrije Universiteit Brussel, Brussel B1050, Belgium.
- 95Department of Obstetrics and Gynecology, University of Melbourne, Victoria 3052, Australia.
- 96Center for Environment and Water, Research Institute, King Fahd University of Petroleum and Minerals, Dhahran 3126, Saudi Arabia .
- 97School of Engineering and Materials Science, Queen Mary University of London, Mile End Road, London, E1 4NS, UK.
- 98Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada.
- 99Department of Pathology, Stony Brook School of Medicine, Stony Brook University, The State University of New York, Stony Brook, NY 11794-8691, USA.
- 100Charles University in Prague, Third Faculty of Medicine, CZ-100 00 Prague 10, Czech Republic.
- 101School of Biological Sciences, The University of Reading, Whiteknights, Reading RG6 6UB, England.
- 102Department of Pathology, Wayne State University, Detroit, MI 48201, USA.
- 103Computer Science Department, Southern Illinois University, Carbondale, IL 62901, USA.
- 104White River Junction Veterans Affairs Medical Center, White River Junction, VT 05009, USA, Geisel School of Medicine at Dartmouth, Hanover, NH 03755, USA.
- 105Human Genetics Unit, Department of Pathology, Faculty of Medicine, Kuwait University, Jabriya 13110, Kuwait.
- 106Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA.
- 107Department of Biological, Chemical, and Pharmaceutical Sciences and Technologies, Polyclinic Plexus, University of Palermo, Palermo 90127, Italy , Sbarro Institute for Cancer Research and Molecular Medicine, Temple University, Philadelphia, PA 19122, USA.
- 108Department of Biological, Chemical, and Pharmaceutical Sciences and Technologies, Polyclinic Plexus, University of Palermo, Palermo 90127, Italy .
- 109Instituto de Alta Investigacion, Universidad de Tarapaca, Arica, Chile.
- 110Department of Pharmacology and Toxicology, University of Vienna, Vienna A-1090, Austria, Institute of Cancer Research, Department of Medicine, Medical University of Vienna, Wien 1090, Austria.
- 111Departments of Pharmacology and Hematology and Medical Oncology, Emory University School of Medicine and Winship Cancer Institute, Atlanta, GA 30322, USA.
- 112Division of Hematology and Oncology, Department of Pediatrics, Children's Healthcare of Atlanta, GA 30322, USA, Department of Pediatrics, Emory University School of Medicine, Emory University, Atlanta, GA 30322, USA.
- 113Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, NC 27599, USA.
- 114Department of Cancer Biology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
- 115Program in Genetics and Molecular Biology, Graduate Division of Biological and Biomedical Sciences, Emory University, Atlanta, GA 30322, USA.
- 116Population Health and Prevention, Research, Prevention and Cancer Control, Cancer Care Ontario, Toronto, Ontario, M5G 2L7, Canada, Departments of Epidemiology and Occupational and Environmental Health, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, M5T 3M7, Canada .
- 117Department of Cardiac, Thoracic and Vascular Sciences, Unit of Occupational Medicine, University of Padova, Padova 35128, Italy.
- 118Department of Anatomy, Physiology and Biochemistry, The Swedish University of Agricultural Sciences, PO Box 7011, VHC, Almas Allé 4, SE-756 51, Uppsala, Sweden.
- 119Siriraj Center of Excellence for Stem Cell Research, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
- 120Department of Anatomy, College of Medicine, Yeungnam University, Daegu 705-717, South Korea.
- 121Department of Hygiene, Kawasaki Medical School, Matsushima Kurashiki, Okayama 701-0192, Japan.
- 122Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, United States Food and Drug Administration, Jefferson, AR 72079, USA.
- 123INSERM U1113, team 3 'Cell Signalling and Communication in Kidney and Prostate Cancer', University of Strasbourg, Faculté de Médecine, 67085 Strasbourg, France.
- 124INRS-Institut Armand-Frappier, 531 Boulevard des Prairies, Laval, QC H7V 1B7, Canada.
- 125Department of Biology, Geology and Environmental Sciences, Alma Mater Studiorum Università di Bologna, Via Francesco Selmi, 3, 40126 Bologna, Italy, Center for Applied Biomedical Research, S. Orsola-Malpighi University Hospital, Via Massarenti, 9, 40126 Bologna, Italy, National Institute of Biostructures and Biosystems, Viale Medaglie d' Oro, 305, 00136 Roma, Italy.
- 126Department of Biosciences and Veterinary Public Health, Faculty of Veterinary Medicine, Swedish University of Agricultural Sciences, PO Box 7028, 75007 Uppsala, Sweden.
- 127INSERM U1113, team 3 'Cell Signalling and Communication in Kidney and Prostate Cancer', University of Strasbourg, Faculté de Médecine, 67085 Strasbourg, France, Department of Cell and Developmental Biology, University of California, Irvine, CA 92697, USA.
- 128Department of Biology/Center for Cancer Research and Therapeutic Development, Clark Atlanta University, Atlanta, GA 30314, USA.
- 129Harvard Medical School/Brigham and Women's Hospital, Boston, MA 02115, USA.
- 130Baylor College of Medicine, Houston, TX 77030, USA.
- 131Department of Pharmaceutical Sciences, West Virginia University, Morgantown, WV, 26506, USA.
- 132Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Kuwait University, PO Box 24923, Safat 13110, Kuwait and.
- 133Department of Surgery, The Ohio State University College of Medicine, The James Comprehensive Cancer Center, Columbus, OH 43210, USA.
Abstract
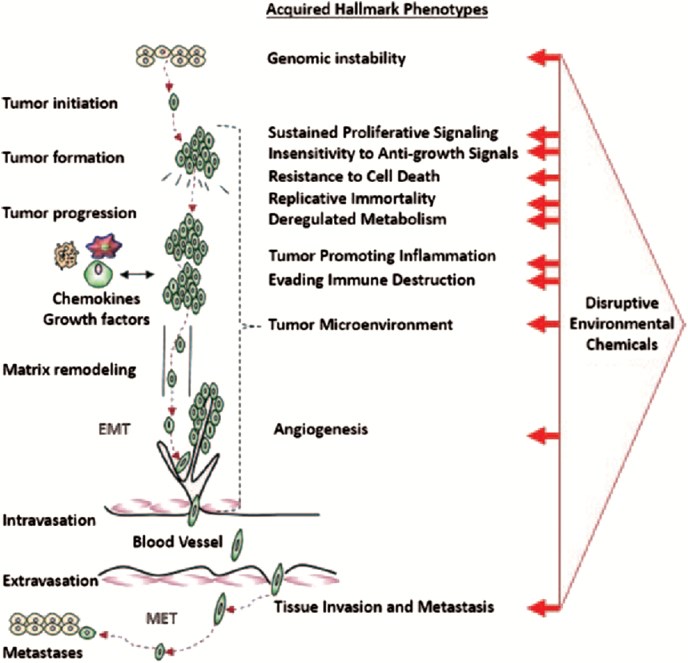
Lifestyle factors are responsible for a considerable portion of cancer incidence worldwide, but credible estimates from the World Health Organization and the International Agency for Research on Cancer (IARC) suggest that the fraction of cancers attributable to toxic environmental exposures is between 7% and 19%. To explore the hypothesis that low-dose exposures to mixtures of chemicals in the environment may be combining to contribute to environmental carcinogenesis, we reviewed 11 hallmark phenotypes of cancer, multiple priority target sites for disruption in each area and prototypical chemical disruptors for all targets, this included dose-response characterizations, evidence of low-dose effects and cross-hallmark effects for all targets and chemicals. In total, 85 examples of chemicals were reviewed for actions on key pathways/mechanisms related to carcinogenesis. Only 15% (13/85) were found to have evidence of a dose-response threshold, whereas 59% (50/85) exerted low-dose effects. No dose-response information was found for the remaining 26% (22/85). Our analysis suggests that the cumulative effects of individual (non-carcinogenic) chemicals acting on different pathways, and a variety of related systems, organs, tissues and cells could plausibly conspire to produce carcinogenic synergies. Additional basic research on carcinogenesis and research focused on low-dose effects of chemical mixtures needs to be rigorously pursued before the merits of this hypothesis can be further advanced. However, the structure of the World Health Organization International Programme on Chemical Safety 'Mode of Action' framework should be revisited as it has inherent weaknesses that are not fully aligned with our current understanding of cancer biology.
Figures
Similar articles
Hu Z, Brooks SA, Dormoy V, Hsu CW, Hsu HY, Lin LT, Massfelder T, Rathmell WK, Xia M, Al-Mulla F, Al-Temaimi R, Amedei A, Brown DG, Prudhomme KR, Colacci A, Hamid RA, Mondello C, Raju J, Ryan EP, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Lowe L, Jensen L, Bisson WH, Kleinstreuer N.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S184-202. doi: 10.1093/carcin/bgv036.PMID: 26106137 Free PMC article. Review.
Disruptive environmental chemicals and cellular mechanisms that confer resistance to cell death.
Narayanan KB, Ali M, Barclay BJ, Cheng QS, D'Abronzo L, Dornetshuber-Fleiss R, Ghosh PM, Gonzalez Guzman MJ, Lee TJ, Leung PS, Li L, Luanpitpong S, Ratovitski E, Rojanasakul Y, Romano MF, Romano S, Sinha RK, Yedjou C, Al-Mulla F, Al-Temaimi R, Amedei A, Brown DG, Ryan EP, Colacci A, Hamid RA, Mondello C, Raju J, Salem HK, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Kim SY, Bisson WH, Lowe L, Park HH.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S89-110. doi: 10.1093/carcin/bgv032.PMID: 26106145 Free PMC article. Review.
Causes of genome instability: the effect of low dose chemical exposures in modern society.
Langie SA, Koppen G, Desaulniers D, Al-Mulla F, Al-Temaimi R, Amedei A, Azqueta A, Bisson WH, Brown DG, Brunborg G, Charles AK, Chen T, Colacci A, Darroudi F, Forte S, Gonzalez L, Hamid RA, Knudsen LE, Leyns L, Lopez de Cerain Salsamendi A, Memeo L, Mondello C, Mothersill C, Olsen AK, Pavanello S, Raju J, Rojas E, Roy R, Ryan EP, Ostrosky-Wegman P, Salem HK, Scovassi AI, Singh N, Vaccari M, Van Schooten FJ, Valverde M, Woodrick J, Zhang L, van Larebeke N, Kirsch-Volders M, Collins AR.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S61-88. doi: 10.1093/carcin/bgv031.PMID: 26106144 Free PMC article. Review.
Engström W, Darbre P, Eriksson S, Gulliver L, Hultman T, Karamouzis MV, Klaunig JE, Mehta R, Moorwood K, Sanderson T, Sone H, Vadgama P, Wagemaker G, Ward A, Singh N, Al-Mulla F, Al-Temaimi R, Amedei A, Colacci AM, Vaccari M, Mondello C, Scovassi AI, Raju J, Hamid RA, Memeo L, Forte S, Roy R, Woodrick J, Salem HK, Ryan EP, Brown DG, Bisson WH.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S38-60. doi: 10.1093/carcin/bgv030.PMID: 26106143 Free PMC article. Review.
Robey RB, Weisz J, Kuemmerle NB, Salzberg AC, Berg A, Brown DG, Kubik L, Palorini R, Al-Mulla F, Al-Temaimi R, Colacci A, Mondello C, Raju J, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Amedei A, Hamid RA, Williams GP, Lowe L, Meyer J, Martin FL, Bisson WH, Chiaradonna F, Ryan EP.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S203-31. doi: 10.1093/carcin/bgv037.PMID: 26106140 Free PMC article. Review.
Cited by
Limbu S, Dakshanamurthy S.Toxics. 2023 Jul 12;11(7):605. doi: 10.3390/toxics11070605.PMID: 37505571 Free PMC article.
A Collaborative Approach to Address Racism in a Community-Academic Partnership.
Lebow-Skelley E, Scott Tomlinson M, Charles S, Fuller C, Ames B, Pearson MA.Prev Chronic Dis. 2023 Jun 8;20:E47. doi: 10.5888/pcd20.220365.PMID: 37290007 Free PMC article.
A complex systems model of breast cancer etiology: The Paradigm II Model.
Hiatt RA, Worden L, Rehkopf D, Engmann N, Troester M, Witte JS, Balke K, Jackson C, Barlow J, Fenton SE, Gehlert S, Hammond RA, Kaplan G, Kornak J, Nishioka K, McKone T, Smith MT, Trasande L, Porco TC.PLoS One. 2023 May 19;18(5):e0282878. doi: 10.1371/journal.pone.0282878. eCollection 2023.PMID: 37205649 Free PMC article.
Burdon J, Budnik LT, Baur X, Hageman G, Howard CV, Roig J, Coxon L, Furlong CE, Gee D, Loraine T, Terry AV Jr, Midavaine J, Petersen H, Bron D, Soskolne CL, Michaelis S.Environ Health. 2023 May 16;22(1):43. doi: 10.1186/s12940-023-00987-8.PMID: 37194087 Free PMC article.
Dinca V, Docea AO, Drocas AI, Nikolouzakis TK, Stivaktakis PD, Nikitovic D, Golokhvast KS, Hernandez AF, Calina D, Tsatsakis A.Arch Toxicol. 2023 May;97(5):1285-1298. doi: 10.1007/s00204-023-03455-x. Epub 2023 Mar 9.PMID: 36892595 Free PMC article.
KMEL References
References
-
- (2014) World cancer report 2014. In Wild C.P. and Stewart B.W (eds). World Health Organization.
-
- Malhotra J. (2014) Molecular and genetic epidemiology of cancer in low- and medium-income countries. Ann. Glob. Health, 80, 418–425. - PubMed
-
- Sankpal U.T., et al. (2012) Environmental factors in causing human cancers: emphasis on tumorigenesis. Tumour Biol., 33, 1265–1274. - PubMed
-
- Trosko J.E., et al. (2005) The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis, 20, 81–92. - PubMed
-
- Christiani D.C. (2011) Combating environmental causes of cancer. N. Engl. J. Med., 364, 791–793. - PubMed
-
- Clapp R. (2011) Chemicals policy in the 2008-2009 President's Cancer Panel Report. New Solut., 21, 447–455. - PubMed
-
- Reuben S.H. (2008–2009) Reducing environmental cancer risk: what we can do now. In Panel T.P.s.C. (ed.), Bethesda, Maryland.
-
- (2009) Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization, Geneva.
-
- Straif K. (2008) The burden of occupational cancer. Occup. Environ. Med., 65, 787–788. - PubMed
-
- (2009) OECD Guidelines for the Testing of Chemicals, Section 4 Health Effects, Test No. 451: Carcinogenicity Studies. OECD.
-
- Ames B.N. (1979) Identifying environmental chemicals causing mutations and cancer. Science, 204, 587–593. - PubMed
-
- Truhaut R. (1990) [Recent progress in the evaluation of the dangers of chemical carcinogens]. J. Pharm. Belg., 45, 131–140. - PubMed
-
- Hanahan D., et al. (2000) The hallmarks of cancer. Cell, 100, 57–70. - PubMed
-
- Preston R.J. (2005) Extrapolations are the Achilles heel of risk assessment. Mutat. Res., 589, 153–157. - PubMed
-
- Hanahan D., et al. (2011) Hallmarks of cancer: the next generation. Cell, 144, 646–674. - PubMed
-
- Colotta F., et al. (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis, 30, 1073–1081. - PubMed
-
- Warburg O. (ed.) (1930) The Metabolism of Tumours: Investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlen. Constable & Company Limited, London.
-
- Aisenberg A.C. (1961) The Glycolysis and Respiration of Tumors. Academic Press, New York, NY.
-
- Ankley G.T., et al. (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem., 29, 730–741. - PubMed
-
- Groh K.J., et al. (2015) Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere, 120, 764–777. - PubMed
-
- Kleinstreuer N.C., et al. (2013) In vitro perturbations of targets in cancer hallmark processes predict rodent chemical carcinogenesis. Toxicol. Sci., 131, 40–55. - PubMed
-
- (2001) National Toxicology Program’s report of the endocrine disruptors low dose peer review. National Institute of Environmental Health Sciences, National Toxicology Program, Research Triangle Park, NC.
-
- Welshons W.V., et al. (2006) Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology, 147(Suppl 6), S56–S69. - PubMed
-
- Vandenberg L.N., et al. (2007) Human exposure to bisphenol A (BPA). Reprod. Toxicol., 24, 139–177. - PubMed
-
- EPA, U.S. The U.S. Environmental Protection Agency ToxCast Phase I/II data http://www.epa.gov/ncct/toxcast/data.html.
-
- Goldman J.M., et al. (2004) Methoxychlor-induced alterations in the histological expression of angiogenic factors in pituitary and uterus. J. Mol. Histol., 35, 363–375. - PubMed
-
- Chapin R.E., et al. (1997) The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam. Appl. Toxicol., 40, 138–157. - PubMed
-
- Hu J.X., et al. (2013) Toxic effects of cypermethrin on the male reproductive system: with emphasis on the androgen receptor. J. Appl. Toxicol., 33, 576–585. - PubMed
-
- Jin M., et al. (2010) Estrogenic activities of two synthetic pyrethroids and their metabolites. J. Environ. Sci. (China), 22, 290–296. - PubMed
-
- Kakko I., et al. (2004) Oestradiol potentiates the effects of certain pyrethroid compounds in the MCF7 human breast carcinoma cell line. Altern. Lab. Anim., 32, 383–390. - PubMed
-
- Luo C., et al. (2013) A cigarette component acrolein induces accelerated senescence in human diploid fibroblast IMR-90 cells. Biogerontology, 14, 503–511. - PubMed
-
- Roy J., et al. (2010) Acrolein induces apoptosis through the death receptor pathway in A549 lung cells: role of p53. Can. J. Physiol. Pharmacol., 88, 353–368. - PubMed
-
- Tanel A., et al. (2014) Acrolein activates cell survival and apoptotic death responses involving the endoplasmic reticulum in A549 lung cells. Biochim. Biophys. Acta, 1843, 827–835. - PubMed
-
- Deng Y.T., et al. (2010) Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog., 49, 141–151. - PubMed
-
- Li Y., et al. (2013) Copper induces cellular senescence in human glioblastoma multiforme cells through downregulation of Bmi-1. Oncol. Rep., 29, 1805–1810. - PubMed
-
- Ostrakhovitch E.A., et al. (2005) Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis, 10, 111–121. - PubMed
-
- Parr-Sturgess C.A., et al. (2012) Copper modulates zinc metalloproteinase-dependent ectodomain shedding of key signaling and adhesion proteins and promotes the invasion of prostate cancer epithelial cells. Mol. Cancer Res., 10, 1282–1293. - PubMed
-
- Freitas M., et al. (2013) Nickel induces apoptosis in human neutrophils. Biometals, 26, 13–21. - PubMed
-
- Yuan D., et al. (2013) Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat. Res., 757, 125–131. - PubMed
-
- Aluigi M.G., et al. (2010) Apoptosis as a specific biomarker of diazinon toxicity in NTera2-D1 cells. Chem. Biol. Interact., 187, 299–303. - PubMed
-
- Giordano G., et al. (2007) Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol., 219, 181–189. - PubMed
-
- Gilsing A.M., et al. (2013) Dietary heme iron and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis, 34, 2757–2766. - PubMed
-
- Pluth J.M., et al. (1996) Increased frequency of specific genomic deletions resulting from in vitro malathion exposure. Cancer Res., 56, 2393–2399. - PubMed
-
- Chen Z.J., et al. (2014) Bisphenol A modulates colorectal cancer protein profile and promotes the metastasis via induction of epithelial to mesenchymal transitions. Arch Toxicol. - PubMed
-
- Zhu H., et al. (2010) Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol. Rep., 23, 129–139. - PubMed
-
- Pontillo C.A., et al. (2013) Action of hexachlorobenzene on tumor growth and metastasis in different experimental models. Toxicol. Appl. Pharmacol., 268, 331–342. - PubMed
-
- Ornstein D.L., et al. (2007) Iron stimulates urokinase plasminogen activator expression and activates NF-kappa B in human prostate cancer cells. Nutr. Cancer, 58, 115–126. - PubMed
-
- Wetherill Y.B., et al. (2002) The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol. Cancer Ther., 1, 515–524. - PubMed
-
- Park S.H., et al. (2009) Cell growth of ovarian cancer cells is stimulated by xenoestrogens through an estrogen-dependent pathway, but their stimulation of cell growth appears not to be involved in the activation of the mitogen-activated protein kinases ERK-1 and p38. J. Reprod. Dev., 55, 23–29. - PubMed
-
- Wilkinson C.F., et al. (1996) A mechanistic interpretation of the oncogenicity of chlorothalonil in rodents and an assessment of human relevance. Regul. Toxicol. Pharmacol., 24(1 Pt 1), 69–84. - PubMed
-
- Vesselinovitch S.D., et al. (1983) Lindane bioassay studies and human cancer risk assessment. Toxicol. Pathol., 11, 12–22. - PubMed
-
- Lee H.R., et al. (2012) Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int. J. Mol. Med., 29, 883–890. - PubMed
-
- Stagg N.J., et al. (2012) Assessment of possible carcinogenicity of oxyfluorfen to humans using mode of action analysis of rodent liver effects. Toxicol. Sci., 128, 334–345. - PubMed
-
- Doull J., et al. (1999) A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul. Toxicol. Pharmacol., 29, 327–357. - PubMed
-
- Mazzoleni G., et al. (1994) Influence of the herbicide Linuron on growth rate and gap-junctional intercellular communication of cultured endothelial cells. J. Environ. Pathol. Toxicol. Oncol., 13, 1–10. - PubMed
-
- Yasaei H., et al. (2013) Carcinogen-specific mutational and epigenetic alterations in INK4A, INK4B and p53 tumour-suppressor genes drive induced senescence bypass in normal diploid mammalian cells. Oncogene, 32, 171–179. - PubMed
-
- Singh K.P., et al. (2008) Allelic loss and mutations in a new ETRG-1 gene are early events in diethylstilbestrol-induced renal carcinogenesis in Syrian hamsters. Gene, 408, 18–26. - PubMed
-
- Tsutsui T., et al. (1994) Reserpine-induced cell transformation without detectable genetic effects in Syrian hamster embryo cells in culture. Carcinogenesis, 15, 11–14. - PubMed
-
- Martens U., et al. (1996) Low expression of the WAF1/CIP1 gene product, p21, in enzyme-altered foci induced in rat liver by diethylnitrosamine or phenobarbital. Cancer Lett., 104, 21–26. - PubMed
-
- Geter D.R., et al. (2014) Dose-response modeling of early molecular and cellular key events in the CAR-mediated hepatocarcinogenesis pathway. Toxicol. Sci., 138, 425–445. - PubMed
-
- Bader A., et al. (2011) Paracetamol treatment increases telomerase activity in rat embryonic liver cells. Pharmacol. Rep., 63, 1435–1441. - PubMed
-
- Tsuruga Y., et al. (2008) Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant., 17, 1083–1094. - PubMed
-
- Nguyen T.H., et al. (2005) Treatment of acetaminophen-induced acute liver failure in the mouse with conditionally immortalized human hepatocytes. J. Hepatol., 43, 1031–1037. - PubMed
-
- Bode-Böger S.M., et al. (2005) Aspirin reduces endothelial cell senescence. Biochem. Biophys. Res. Commun., 334, 1226–1232. - PubMed
-
- Heinloth A.N., et al. (2004) Gene expression profiling of rat livers reveals indicators of potential adverse effects. Toxicol. Sci., 80, 193–202. - PubMed
-
- Jacob T., et al. (2009) The effect of cotinine on telomerase activity in human vascular smooth muscle cells. J. Cardiovasc. Surg. (Torino), 50, 345–349. - PubMed
-
- Brüne B., et al. (2001) Transcription factors p53 and HIF-1alpha as targets of nitric oxide. Cell. Signal., 13, 525–533. - PubMed
-
- Davis C.D., et al. (2000) Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J. Nutr., 130, 2903–2909. - PubMed
-
- Arnér E.S., et al. (2006) The thioredoxin system in cancer. Semin. Cancer Biol., 16, 420–426. - PubMed
-
- Qin X.Y., et al. (2012) Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol. Ther., 13, 296–306. - PubMed
-
- Peluso M.E., et al. (2014) Bisphenol-A exposures and behavioural aberrations: median and linear spline and meta-regression analyses of 12 toxicity studies in rodents. Toxicology, 325, 200–208. - PubMed
-
- Fang C.C., et al. (2013) Cyprodinil as an activator of aryl hydrocarbon receptor. Toxicology, 304, 32–40. - PubMed
-
- Bharadwaj R., et al. (2004) The spindle checkpoint, aneuploidy, and cancer. Oncogene, 23, 2016–2027. - PubMed
-
- Tanaka T., et al. (2013) Effects of maternal exposure to imazalil on behavioral development in F₁-generation mice. Birth Defects Res. B Dev. Reprod. Toxicol., 98, 334–342. - PubMed
-
- Ahmad I., et al. (2008) The involvement of nitric oxide in maneb- and paraquat-induced oxidative stress in rat polymorphonuclear leukocytes. Free Radic. Res., 42, 849–862. - PubMed
-
- US Environmental Protection Agency (1988) US Integrated Risk Information System—Maneb (CASRN 12427-38-2). http://www.epa.gov/iris/subst/0249.htm .
-
- Miller K.P., et al. (2006) Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol. Sci., 93, 180–188. - PubMed
-
- Palanza P., et al. (2001) Effects of prenatal exposure to low doses of diethylstilbestrol, o,p'DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm. Behav., 40, 252–265. - PubMed
-
- Du G., et al. (2013) Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo . Environ. Toxicol. Chem., 32, 353–360. - PubMed
-
- Kim H.S., et al. (2011) Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. J. Toxicol. Sci., 36, 201–210. - PubMed
-
- Eveillard A., et al. (2009) Di-(2-ethylhexyl)-phthalate (DEHP) activates the constitutive androstane receptor (CAR): a novel signalling pathway sensitive to phthalates. Biochem. Pharmacol., 77, 1735–1746. - PubMed
-
- Nakai M., et al. (1999) Binding characteristics of dialkyl phthalates for the estrogen receptor. Biochem. Biophys. Res. Commun., 254, 311–314. - PubMed
-
- Grande S.W., et al. (2006) A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol. Sci., 91, 247–254. - PubMed
-
- Kojima H., et al. (2011) Comparative study of human and mouse pregnane X receptor agonistic activity in 200 pesticides using in vitro reporter gene assays. Toxicology, 280, 77–87. - PubMed
-
- (2006) Phosalone Reregistration Eligibility Decision (RED). The United States Environmental Protection Agency Office of Pesticide Programs.
-
- Li X., et al. (2013) Structure-dependent activities of hydroxylated polybrominated diphenyl ethers on human estrogen receptor. Toxicology, 309, 15–22. - PubMed
-
- Berger R.G., et al. (2014) Exposure to an environmentally relevant mixture of brominated flame retardants affects fetal development in Sprague-Dawley rats. Toxicology, 320, 56–66. - PubMed
-
- Hofmeister M.V., et al. (2004) Effects of the pesticides prochloraz and methiocarb on human estrogen receptor alpha and beta mRNA levels analyzed by on-line RT-PCR. Toxicol. In Vitro, 18, 427–433. - PubMed
-
- Jacobsen P.R., et al. (2012) Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol., 34, 237–250. - PubMed
-
- Kamanga-Sollo E., et al. (2008) Roles of IGF-I and the estrogen, androgen and IGF-I receptors in estradiol-17beta- and trenbolone acetate-stimulated proliferation of cultured bovine satellite cells. Domest. Anim. Endocrinol., 35, 88–97. - PubMed
-
- Yarrow J.F., et al. (2010) Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids, 75, 377–389. - PubMed
-
- Erden E.S., et al. (2014) Investigation of Bisphenol A as an endocrine disruptor, total thiol, malondialdehyde, and C-reactive protein levels in chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci., 18, 3477–3483. - PubMed
-
- Liu Y., et al. (2014) Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical Bisphenol-A. Biochem. Biophys. Res. Commun., 451, 592–598. - PubMed
-
- Rogers J.A., et al. (2013) Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol., 53, 421–430. - PubMed
-
- Koike E., et al. (2014) Penta- and octa-bromodiphenyl ethers promote proinflammatory protein expression in human bronchial epithelial cells in vitro . Toxicol. In Vitro, 28, 327–333. - PubMed
-
- Zhao S., et al. (2013) Sub-acute exposure to the herbicide atrazine suppresses cell immune functions in adolescent mice. Biosci. Trends, 7, 193–201. - PubMed
-
- Zhou H.R., et al. (2003) Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci., 72, 130–142. - PubMed
-
- Shin S.G., et al. (2005) Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression in RAW 264.7 macrophages by sesquiterpene lactones. J. Toxicol. Environ. Health A, 68, 2119–2131. - PubMed
-
- (1997) BASF Corporation Pyridaben (Sanmite) Pesticide Tolerance Petition 3/97, US EPA [PF-721; FRL-5592 -7], http//pmep.cce.cornell. edu/profiles/insect-mite/mevinphos-propargite/py... (accessed 7 May 2015)
-
- Barros S.P., et al. (2010) Triclosan inhibition of acute and chronic inflammatory gene pathways. J. Clin. Periodontol., 37, 412–418. - PubMed
-
- Bhargava H.N., et al. (1996) Triclosan: applications and safety. Am. J. Infect. Control, 24, 209–218. - PubMed
-
- Stoker T.E., et al. (2010) Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci., 117, 45–53. - PubMed
-
- Welshons W.V., et al. (1999) Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol. Ind. Health, 15, 12–25. - PubMed
-
- Jones B.A., et al. (2011) Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm. Behav., 59, 246–251. - PubMed
-
- Lemos M.F., et al. (2010) Protein differential expression induced by endocrine disrupting compounds in a terrestrial isopod. Chemosphere, 79, 570–576. - PubMed
-
- Filipov N.M., et al. (2005) Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B–dependent mechanism. Toxicol. Sci., 84, 139–148. - PubMed
-
- Knudsen T.B., et al. (2011) Disruption of embryonic vascular development in predictive toxicology. Birth Defects Res. Part C, 93, 312–323. - PubMed
-
- Qin R., et al. (2011) Protection by tetrahydroxystilbene glucoside against neurotoxicity induced by MPP+: the involvement of PI3K/Akt pathway activation. Toxicol. Lett., 202, 1–7. - PubMed
-
- Manfo F.P., et al. (2011) Effects of maneb on testosterone release in male rats. Drug Chem. Toxicol., 34, 120–128. - PubMed
-
- Matsushita T., et al. (1976) Experimental study on contact dermatitis caused by dithiocarbamates maneb, mancozeb, zineb, and their related compounds. Int. Arch. Occup. Environ. Health, 37, 169–178. - PubMed
-
- Barlow, B. et al. (2005) Modulation of antioxidant defense systems by the environmental pesticide Maneb in dopaminergic cells. Neurotoxicol., 26, 63–75 . - PubMed
-
- Kazantseva Y.A., et al. (2013) Dichlorodiphenyltrichloroethane technical mixture regulates cell cycle and apoptosis genes through the activation of CAR and ERα in mouse livers. Toxicol. Appl. Pharmacol., 271, 137–143. - PubMed
-
- Lin Z.X., et al. (1986) Inhibition of gap junctional intercellular communication in human teratocarcinoma cells by organochlorine pesticides. Toxicol. Appl. Pharmacol., 83, 10–19. - PubMed
-
- Ruch R.J., et al. (1987) Inhibition of intercellular communication between mouse hepatocytes by tumor promoters. Toxicol. Appl. Pharmacol., 87, 111–120. - PubMed
-
- Ventura C., et al. (2012) Differential mechanisms of action are involved in chlorpyrifos effects in estrogen-dependent or -independent breast cancer cells exposed to low or high concentrations of the pesticide. Toxicol. Lett., 213, 184–193. - PubMed
-
- Mense S.M., et al. (2006) The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci., 93, 125–135. - PubMed
-
- Santucci M.A., et al. (2003) Cell-cycle deregulation in BALB/c 3T3 cells transformed by 1,2-dibromoethane and folpet pesticides. Environ. Mol. Mutagen., 41, 315–321. - PubMed
-
- Tsuda H., et al. (2005) High susceptibility of human c-Ha-ras proto-oncogene transgenic rats to carcinogenesis: a cancer-prone animal model. Cancer Sci., 96, 309–316. - PubMed
-
- Wetzel L.T., et al. (1994) Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J. Toxicol. Environ. Health, 43, 169–182. - PubMed
-
- Andersson H., et al. (2012) Proangiogenic effects of environmentally relevant levels of bisphenol A in human primary endothelial cells. Arch. Toxicol., 86, 465–474. - PubMed
-
- Andrysík Z., et al. (2013) Aryl hydrocarbon receptor-mediated disruption of contact inhibition is associated with connexin43 downregulation and inhibition of gap junctional intercellular communication. Arch. Toxicol., 87, 491–503. - PubMed
-
- Haber L.T., et al. (2000) Hazard identification and dose response of inhaled nickel-soluble salts. Regul. Toxicol. Pharmacol., 31, 210–230. - PubMed
-
- LN V., et al. (2013) Low dose effects of bisphenol A: an integrated review of in vitro, laboratory animal and human studies. Endocrine Disruptors, 1, e1.1–e1.20.
-
- Tryphonas H., et al. (2004) Oral (gavage), in utero and post-natal exposure of Sprague-Dawley rats to low doses of tributyltin chloride. Part II: effects on the immune system. Food Chem. Toxicol., 42, 221–235. - PubMed
-
- Watanabe J., et al. (2013) Low dose of methylmercury (MeHg) exposure induces caspase mediated-apoptosis in cultured neural progenitor cells. J. Toxicol. Sci., 38, 931–935. - PubMed
-
- Petroni D., et al. (2012) Low-dose methylmercury-induced oxidative stress, cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma (SH-SY5Y) cells. Environ. Toxicol., 27, 549–555. - PubMed
-
- McCormack A.L., et al. (2005) Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J. Neurochem., 93, 1030–1037. - PubMed
-
- Asmuss M., et al. (2000) Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis, 21, 2097–2104. - PubMed
-
- Zhang X., et al. (2013) Environmental and occupational exposure to chemicals and telomere length in human studies. Occup. Environ. Med., 70, 743–749. - PubMed
-
- Exon J.H. (2006) A review of the toxicology of acrylamide. J. Toxicol. Environ. Health B. Crit. Rev., 9, 397–412. - PubMed
-
- Sickles D.W., et al. (2007) Acrylamide effects on kinesin-related proteins of the mitotic/meiotic spindle. Toxicol. Appl. Pharmacol., 222, 111–121. - PubMed
-
- Wang X., et al. (2013) Epigenotoxicity of environmental pollutants evaluated by a combination of DNA methylation inhibition and capillary electrophoresis-laser-induced fluorescence immunoassay. Anal. Bioanal. Chem., 405, 2435–2442. - PubMed
-
- Roedel E.Q., et al. (2012) Pulmonary toxicity after exposure to military-relevant heavy metal tungsten alloy particles. Toxicol. Appl. Pharmacol., 259, 74–86. - PubMed
-
- Freyre-Fonseca V., et al. (2011) Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicol. Lett., 202, 111–119. - PubMed
-
- Elhajouji A., et al. (2011) Potential thresholds for genotoxic effects by micronucleus scoring. Mutagenesis, 26, 199–204. - PubMed
-
- Muller J., et al. (2008) Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis, 29, 427–433. - PubMed
-
- Santella R.M., et al. (2005) DNA adducts, DNA repair genotype/phenotype and cancer risk. Mutat. Res., 592, 29–35. - PubMed
-
- (2011) Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. International Atomic Energy Agency, Vienna.
-
- De Lange T. (2005) Telomere-related genome instability in cancer. Cold Spring Harb. Symp. Quant. Biol., 70, 197–204. - PubMed
-
- Frias C., et al. (2012) Telomere dysfunction and genome instability. Front. Biosci. (Landmark Ed), 17, 2181–2196. - PubMed
-
- Hollstein M., et al. (1991) p53 mutations in human cancers. Science, 253, 49–53. - PubMed
-
- Muñoz D.M., et al. (2013) Loss of p53 cooperates with K-ras activation to induce glioma formation in a region-independent manner. Glia, 61, 1862–1872. - PubMed
-
- Pierotti, M.A. et al. Mechanisms of oncogene activation. Kufe DW, Pollock RE, Weichselbaum RR, and et al. Holland-Frei Cancer Medicine. 6th. 2003. Hamilton (ON), BC Decker .
-
- Mazzei F., et al. (2013) Role of MUTYH in human cancer. Mutat. Res., 743–744, 33–43. - PubMed
-
- Sancar A. (1995) Excision repair in mammalian cells. J. Biol. Chem., 270, 15915–15918. - PubMed
-
- Vineis P., et al. (2009) A field synopsis on low-penetrance variants in DNA repair genes and cancer susceptibility. J. Natl Cancer Inst., 101, 24–36. - PubMed
-
- Esteller M. (2007) Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet., 8, 286–298. - PubMed
-
- Caffarelli E., et al. (2011) Epigenetic regulation in cancer development. Front. Biosci. (Landmark Ed), 16, 2682–2694. - PubMed
-
- Leyns L., et al. (2012) Genomic integrity of mouse embryonic stem cells. In Embryogenesis. Intech. pp. 333–358.
-
- Blessing H., et al. (2004) Interaction of selenium compounds with zinc finger proteins involved in DNA repair. Eur. J. Biochem., 271, 3190–3199. - PubMed
-
- Zhang X., et al. (2013) Environmental and occupational exposure to chemicals and telomere length in human studies. Postgrad. Med. J., 89, 722–728. - PubMed
-
- Lombaert N., et al. (2013) Hard-metal (WC-Co) particles trigger a signaling cascade involving p38 MAPK, HIF-1α, HMOX1, and p53 activation in human PBMC. Arch. Toxicol., 87, 259–268. - PubMed
-
- Jugan M.L., et al. (2012) Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology, 6, 501–513. - PubMed
-
- Doshi T., et al. (2011) Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology, 289, 74–82. - PubMed
-
- Ribeiro-Varandas E., et al. (2013) Bisphenol A at concentrations found in human serum induces aneugenic effects in endothelial cells. Mutat. Res., 751, 27–33. - PubMed
-
- Marshall H. (2002) Fact sheet: carbendazim. Pesticides News, 57, 20–21.
-
- Zhao Y., et al. (2010) Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal. Chem., 82, 4599–4605. - PubMed
-
- Piao M.J., et al. (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett., 201, 92–100. - PubMed
-
- Gong C., et al. (2012) Methylation of PARP-1 promoter involved in the regulation of nano-SiO2-induced decrease of PARP-1 mRNA expression. Toxicol. Lett., 209, 264–269. - PubMed
-
- Choi A.O., et al. (2008) Quantum dot-induced epigenetic and genotoxic changes in human breast cancer cells. J. Mol. Med. (Berl), 86, 291–302. - PubMed
-
- Balansky R., et al. (2013) Transplacental clastogenic and epigenetic effects of gold nanoparticles in mice. Mutat. Res., 751–752, 42–48. - PubMed
-
- Liu Y., et al. (2013) Understanding the toxicity of carbon nanotubes. Acc. Chem. Res., 46, 702–713. - PubMed
-
- Chisholm H. (1910–1911) 11th Edition of Encyclopedia Britannica. Cambridge University Press, Cambridge, UK.
-
- Stoker T.E., et al. (1999) Prepubertal exposure to compounds that increase prolactin secretion in the male rat: effects on the adult prostate. Biol. Reprod., 61, 1636–1643. - PubMed
-
- Riu A., et al. (2011) Characterization of novel ligands of ERalpha, Erbeta, and PPARgamma: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol. Sci, 122, 372–82. - PubMed
-
- Hooghe R.J., et al. (2000) Effects of selected herbicides on cytokine production in vitro . Life Sci., 66, 2519–2525. - PubMed
-
- Filipov N.M., et al. (2005) Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci., 86, 324–332. - PubMed
-
- Karrow N.A., et al. (2005) Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice. Toxicology, 209, 15–28. - PubMed
-
- Basini G., et al. (2012) Atrazine disrupts steroidogenesis, VEGF and NO production in swine granulosa cells. Ecotoxicol. Environ. Saf., 85, 59–63. - PubMed
-
- Chen J.Y., et al. (2013) Immunotoxicity of atrazine in Balb/c mice. J. Environ. Sci. Health B., 48, 637–645. - PubMed
-
- Chen J., et al. (2015) Effects of atrazine on the proliferation and cytotoxicity of murine lymphocytes with the use of carboxyfluorescein succinimidyl ester-based flow cytometric approaches. Food Chem. Toxicol., 76, 61–69. - PubMed
-
- Danelli L., et al. (2015) Mast cells boost myeloid-derived suppressor cell activity and contribute to the development of tumor-favoring microenvironment. Cancer Immunol. Res., 3, 85–95. - PubMed
-
- Costa A., et al. (2014) The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol., 25, 23–32. - PubMed
-
- Lei Y., et al. (2015) Redox regulation of inflammation: old elements, a new story. Med. Res. Rev., 35, 306–340. - PubMed
-
- Zhang H.Y., et al. (2014) Perinatal exposure to 4-nonylphenol affects adipogenesis in first and second generation rats offspring. Toxicol. Lett., 225, 325–332. - PubMed
-
- Mitchison J. (1971) The Biology of the Cell Cycle. Cambridge University Press.
-
- Keating M.T., et al. (1988) Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science, 239, 914–916. - PubMed
-
- Kerkhoff E., et al. (1998) Cell cycle targets of Ras/Raf signalling. Oncogene, 17, 1457–1462. - PubMed
-
- Grünfeld H.T., et al. (2004) Effect of in vitro estrogenic pesticides on human oestrogen receptor alpha and beta mRNA levels. Toxicol. Lett., 151, 467–480. - PubMed
-
- Symonds D.A., et al. (2005) Methoxychlor induces proliferation of the mouse ovarian surface epithelium. Toxicol. Sci., 83, 355–362. - PubMed
-
- Murono E.P., et al. (2004) The effects of the reported active metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, on testosterone formation by cultured Leydig cells from young adult rats. Reprod. Toxicol., 19, 135–146. - PubMed
-
- Gaido K.W., et al. (2000) Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol. Pharmacol., 58, 852–858. - PubMed
-
- Wilard S, et al. (2009) Growth factors differentially augment the effects of HPTE on estrogen response element-mediated gene transcription in a dose- and time-dependent manner among human breast cancer cell lines. Res. J. Med. Med. Sci. 4, 171–180.
-
- Kojima H., et al. (2010) Endocrine-disrupting potential of pesticides via nuclear receptors and aryl hydrocarbon receptor. J. Health Sci., 56, 374–386.
-
- Noriega N.C., et al. (2005) Late gestational exposure to the fungicide prochloraz delays the onset of parturition and causes reproductive malformations in male but not female rat offspring. Biol. Reprod., 72, 1324–1335. - PubMed
-
- Cocco P. (2002) On the rumors about the silent spring. Review of the scientific evidence linking occupational and environmental pesticide exposure to endocrine disruption health effects. Cad. Saude. Publica., 18, 379–402. - PubMed
-
- Kleinstreuer N.C., et al. (2011) Identifying developmental toxicity pathways for a subset of ToxCast chemicals using human embryonic stem cells and metabolomics. Toxicol. Appl. Pharmacol., 257, 111–121. - PubMed
-
- Cummings A.M., et al. (1989) Antifertility effect of methoxychlor in female rats: dose- and time-dependent blockade of pregnancy. Toxicol. Appl. Pharmacol., 97, 454–462. - PubMed
-
- Gray L.E., Jr, et al. (1989) A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam. Appl. Toxicol., 12, 92–108. - PubMed
-
- Metcalf J.L., et al. (1996) Methoxychlor mimics the action of 17 beta-estradiol on induction of uterine epidermal growth factor receptors in immature female rats. Reprod. Toxicol., 10, 393–399. - PubMed
-
- Kuiper G.G., et al. (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology, 139, 4252–4263. - PubMed
-
- Andersen H.R., et al. (2002) Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro . Toxicol. Appl. Pharmacol., 179, 1–12. - PubMed
-
- Vinggaard A.M., et al. (2006) Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int. J. Androl., 29, 186–192. - PubMed
-
- Liu C., et al. (2011) Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ. Sci. Technol., 45, 769–775. - PubMed
-
- Zhang W., et al. (2013) Known and emerging factors modulating estrogenic effects of estrogen-disrupting chemicals. Environ. Rev., 21, 1–12.
-
- Medjakovic S., et al. (2014) Effect of nonpersistent pesticides on estrogen receptor, androgen receptor, and aryl hydrocarbon receptor. Environ. Toxicol., 29, 1201–1216. - PubMed
-
- Meironyté D., et al. (1999) Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J. Toxicol. Environ. Health A, 58, 329–341. - PubMed
-
- Brown D.J., et al. (2004) Analysis of Ah receptor pathway activation by brominated flame retardants. Chemosphere, 55, 1509–1518. - PubMed
-
- Hsieh T.H., et al. (2012) Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. FASEB J., 26, 778–787. - PubMed
-
- Janjua N.R., et al. (2007) Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol., 41, 5564–5570. - PubMed
-
- Liu W.L., et al. (2010) [Distribution characteristics of phthalic acid esters in soils and plants at e-waste recycling sites in Taizhou of Zhejiang, China]. Ying Yong Sheng Tai Xue Bao, 21, 489–494. - PubMed
-
- Wormuth M., et al. (2006) What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal., 26, 803–824. - PubMed
-
- Galbraith H. (2002) Hormones in international meat production: biological, sociological and consumer issues. Nutr. Res. Rev., 15, 293–314. - PubMed
-
- Boettcher M., et al. (2011) Low-dose effects and biphasic effect profiles: is trenbolone a genotoxicant? Mutat. Res., 723, 152–157. - PubMed
-
- Kongsuwan K., et al. (2012) The effect of combination treatment with trenbolone acetate and estradiol-17β on skeletal muscle expression and plasma concentrations of oxytocin in sheep. Domest. Anim. Endocrinol., 43, 67–73. - PubMed
-
- Hotchkiss A.K., et al. (2007) An environmental androgen, 17beta-trenbolone, affects delayed-type hypersensitivity and reproductive tissues in male mice. J. Toxicol. Environ. Health A, 70, 138–140. - PubMed
-
- Ansari K.M., et al. (2010) Skin tumor promotion by argemone oil/alkaloid in mice: evidence for enhanced cell proliferation, ornithine decarboxylase, cyclooxygenase-2 and activation of MAPK/NF-kappaB pathway. Food Chem. Toxicol., 48, 132–138. - PubMed
-
- Mishra V., et al. (2012) Role of ErbB2 mediated AKT and MAPK pathway in gall bladder cell proliferation induced by argemone oil and butter yellow. Argemone oil and butter yellow induced gall bladder cell proliferation. Cell Biol. Toxicol., 28, 149–159. - PubMed
-
- Parker M. (1991) Nuclear Hormone Receptors: Molecular Mechanisms, Cellular Functions, Clinical Abnormalities. Academic Press, London.
-
- Gulliver L.S.M. (2013) Estradiol synthesis and metabolism and risk of ovarian cancer in older women taking prescribed or plant-derived estrogen supplementation. J. Steroids Horm. Sci., S12:003.
-
- Leroy B., et al. (2014) TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum. Mutat., 35, 672–688. - PubMed
-
- De Blasio A., et al. (2005) Differentiative pathway activated by 3-aminobenzamide, an inhibitor of PARP, in human osteosarcoma MG-63 cells. FEBS Lett., 579, 615–620. - PubMed
-
- Pietruszewska W., et al. (2008) Loss of heterozygosity for Rb locus and pRb immunostaining in laryngeal cancer: a clinicopathologic, molecular and immunohistochemical study. Folia Histochem. Cytobiol., 46, 479–485. - PubMed
-
- Ikushima H., et al. (2010) TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer, 10, 415–424. - PubMed
-
- Campos-Pereira F.D., et al. (2012) Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: a morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol. Environ. Saf., 78, 170–177. - PubMed
-
- Stenner-Liewen F., et al. (2003) Apoptosis and cancer: basic mechanisms and therapeutic opportunities in the postgenomic era. Cancer Res., 63, 263–268.
-
- Thompson C.B. (1995) Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456–1462. - PubMed
-
- Alberts B., et al. (2002) Extracellular control of cell division, cell growth, and apoptosis. In Molecular Biology of the Cell. Garland Science, New York, NY.
-
- Roos W.P., et al. (2006) DNA damage-induced cell death by apoptosis. Trends Mol. Med., 12, 440–450. - PubMed
-
- Fridman J.S., et al. (2003) Control of apoptosis by p53. Oncogene, 22, 9030–9040. - PubMed
-
- Adams J.M. (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev., 17, 2481–2495. - PubMed
-
- Deveraux Q.L., et al. (1999) IAP family proteins–suppressors of apoptosis. Genes Dev., 13, 239–252. - PubMed
-
- Yang Y.L., et al. (2000) The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res., 10, 169–177. - PubMed
-
- Wu W., et al. (2013) Metabolic changes in cancer: beyond the Warburg effect. Acta Biochim. Biophys. Sin. (Shanghai), 45, 18–26. - PubMed
-
- Gonzalez M.J., et al. (2012) The bio-energetic theory of carcinogenesis. Med. Hypotheses, 79, 433–439. - PubMed
-
- Ferreira L.M., et al. (2012) Metabolic reprogramming of the tumor. Oncogene, 31, 3999–4011. - PubMed
-
- Wu G.S. (2009) TRAIL as a target in anti-cancer therapy. Cancer Lett., 285, 1–5. - PubMed
-
- Klaunig J.E., et al. (1990) Gap-junctional intercellular communication and murine hepatic carcinogenesis. Prog. Clin. Biol. Res., 331, 277–291. - PubMed
-
- Carette D., et al. (2014) Connexin a check-point component of cell apoptosis in normal and physiopathological conditions. Biochimie, 101, 1–9. - PubMed
-
- Leung-Toung R., et al. (2006) Thiol proteases: inhibitors and potential therapeutic targets. Curr. Med. Chem., 13, 547–581. - PubMed
-
- Kim I.Y., et al. (2004) Phthalates inhibit tamoxifen-induced apoptosis in MCF-7 human breast cancer cells. J. Toxicol. Environ. Health A, 67, 2025–2035. - PubMed
-
- Corcelle E., et al. (2006) Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer Res., 66, 6861–6870. - PubMed
-
- Kim J.Y., et al. (2014) Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ. Toxicol. Pharmacol., 37, 1264–1274. - PubMed
-
- Carnero A. (2013) Markers of cellular senescence. Methods Mol. Biol., 965, 63–81. - PubMed
-
- Serrano M., et al. (2001) Putting the stress on senescence. Curr. Opin. Cell Biol., 13, 748–753. - PubMed
-
- Shay J.W., et al. (2004) Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene, 23, 2919–2933. - PubMed
-
- Ohtani N., et al. (2004) The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J. Med. Invest., 51, 146–153. - PubMed
-
- Sherr C.J., et al. (2002) The RB and p53 pathways in cancer. Cancer Cell, 2, 103–112. - PubMed
-
- Vergel M., et al. (2010) Bypassing cellular senescence by genetic screening tools. Clin. Transl. Oncol., 12, 410–417. - PubMed
-
- Zanella F., et al. (2010) Understanding FOXO, new views on old transcription factors. Curr. Cancer Drug Targets, 10, 135–146. - PubMed
-
- Newbold R.F., et al. (1982) Induction of immortality is an early event in malignant transformation of mammalian cells by carcinogens. Nature, 299, 633–635. - PubMed
-
- Russo I., et al. (1998) A telomere-independent senescence mechanism is the sole barrier to Syrian hamster cell immortalization. Oncogene, 17, 3417–3426. - PubMed
-
- Newbold R.F., et al. (1980) Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature, 283, 596–599. - PubMed
-
- Lehman T.A., et al. (1993) p53 mutations in human immortalized epithelial cell lines. Carcinogenesis, 14, 833–839. - PubMed
-
- Lafarge-Frayssinet C., et al. (1989) Over expression of proto-oncogenes: ki-ras, fos and myc in rat liver cells treated in vitro by two liver tumor promoters: phenobarbital and biliverdin. Cancer Lett., 44, 191–198. - PubMed
-
- Trott D.A., et al. (1995) Mechanisms involved in the immortalization of mammalian cells by ionizing radiation and chemical carcinogens. Carcinogenesis, 16, 193–204. - PubMed
-
- Rivedal E., et al. (2000) Morphological transformation and effect on gap junction intercellular communication in Syrian hamster embryo cells as screening tests for carcinogens devoid of mutagenic activity. Toxicol. In Vitro, 14, 185–192. - PubMed
-
- Creton S., et al. (2010) Acute toxicity testing of chemicals-Opportunities to avoid redundant testing and use alternative approaches. Crit. Rev. Toxicol., 40, 50–83. - PubMed
-
- MEDES G., et al. (1953) Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro . Cancer Res., 13, 27–29. - PubMed
-
- Menendez J.A., et al. (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer, 7, 763–777. - PubMed
-
- Vander Heiden M.G., et al. (2011) Metabolic pathway alterations that support cell proliferation. Cold Spring Harb. Symp. Quant. Biol., 76, 325–334. - PubMed
-
- Lazebnik Y. (2010) What are the hallmarks of cancer? Nat. Rev. Cancer, 10, 232–233. - PubMed
-
- Berridge M.V., et al. (2010) Metabolic flexibility and cell hierarchy in metastatic cancer. Mitochondrion, 10, 584–588. - PubMed
-
- Floor S.L., et al. (2012) Hallmarks of cancer: of all cancer cells, all the time? Trends Mol. Med., 18, 509–515. - PubMed
-
- Newsholme E.A., et al. (1973) Regulation in Metabolism. Wiley, London, New York.
-
- Fantin V.R., et al. (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell, 9, 425–434. - PubMed
-
- Lapuente-Brun E., et al. (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science, 340, 1567–1570. - PubMed
-
- Weidberg H., et al. (2009) Lipophagy: selective catabolism designed for lipids. Dev. Cell, 16, 628–630. - PubMed
-
- Newsholme E.A., et al. (1991) Application of metabolic-control logic to fuel utilization and its significance in tumor cells. Adv. Enzyme Regul., 31, 225–246. - PubMed
-
- Cho H.M., et al. (2000) Nucleotide sequence and differential expression of the human 3-phosphoglycerate dehydrogenase gene. Gene, 245, 193–201. - PubMed
-
- Owen O.E., et al. (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem., 277, 30409–30412. - PubMed
-
- Meyerhof O. (1951) Mechanisms of glycolysis and fermentation. Can. J. Med. Sci., 29, 63–77. - PubMed
-
- Lowry O.H., et al. (1964) The relationships between substrates and enzymes of glycolysis in brain. J. Biol. Chem., 239, 31–42. - PubMed
-
- Warburg O., et al. (1958) [Partial anaerobiosis and radiation-sensitivity of cancer cells]. Arch. Biochem. Biophys., 78, 573–586. - PubMed
-
- Robey R.B. (2011) On dogma and the metabolic gestalt of tumor cells, Science, E-Letters. https://www.sciencemag.org/content/330/ 6009/1338/reply (7 May 2015, date last accessed).
-
- Copley S.D. (2003) Enzymes with extra talents: moonlighting functions and catalytic promiscuity. Curr. Opin. Chem. Biol., 7, 265–272. - PubMed
-
- Robey R.B., et al. (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene, 25, 4683–4696. - PubMed
-
- Kacser H., et al. (1973) The control of flux. Symp. Soc. Exp. Biol., 27, 65–104. - PubMed
-
- Agarwal A.R., et al. (2013) Metabolic shift in lung alveolar cell mitochondria following acrolein exposure. Am. J. Physiol. Lung Cell. Mol. Physiol., 305, L764–L773. - PubMed
-
- George J., et al. (2011) Cypermethrin exposure leads to regulation of proteins expression involved in neoplastic transformation in mouse skin. Proteomics, 11, 4411–4421. - PubMed
-
- Tsitsimpikou C., et al. (2013) Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology, 307, 109–114. - PubMed
-
- Abdollahi M., et al. (2004) Pesticides and oxidative stress: a review. Med. Sci. Monit., 10, RA141–RA147. - PubMed
-
- Folkman J. (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med., 1, 27–31. - PubMed
-
- Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature, 438, 932–936. - PubMed
-
- Folkman J. (1971) Tumor angiogenesis: therapeutic implications. N. Engl. J. Med., 285, 1182–1186. - PubMed
-
- Ferrara N., et al. (2005) Angiogenesis as a therapeutic target. Nature, 438, 967–974. - PubMed
-
- Folkman J. (2003) Fundamental concepts of the angiogenic process. Curr. Mol. Med., 3, 643–651. - PubMed
-
- Folkman J. (2003) Angiogenesis inhibitors: a new class of drugs. Cancer Biol. Ther., 2, S127–S133. - PubMed
-
- Siemann D.W., et al. (2005) Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res., 11(2 Pt 1), 416–420. - PubMed
-
- Thorpe P.E. (2004) Vascular targeting agents as cancer therapeutics. Clin. Cancer Res., 10, 415–427. - PubMed
-
- Patterson D.M., et al. (2007) Vascular damaging agents. Clin. Oncol. (R. Coll. Radiol.), 19, 443–456. - PubMed
-
- Konigsberg W.H., et al. (1988) Molecular cloning of the cDNA for human tissue factor. Cell, 52, 639–640. - PubMed
-
- Contrino J., et al. (1996) In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat. Med., 2, 209–215. - PubMed
-
- Hu Z. (2011) Factor VII-Targeted Photodynamic Therapy for Breast Cancer and Its Therapeutic Potential for Other Solid Cancers and Leukemia, Breast Cancer—Current and Alternative Therapeutic Modalities. In EsraGunduz and MehmetGunduz (eds), InTech, Rijeka, Croatia. http://www.intechopen.com/articles/show/title/factor-vii-targeted-photod... ISBN: 978-953-307-776-5.
-
- Yang J., et al. (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell, 14, 818–829. - PubMed
-
- Kawata M., et al. (2012) TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J. Biochem., 151, 205–216. - PubMed
-
- Nagase H., et al. (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res., 69, 562–573. - PubMed
-
- Chen H.C., et al. (1998) Tyrosine phosphorylation of focal adhesion kinase stimulated by hepatocyte growth factor leads to mitogen-activated protein kinase activation. J. Biol. Chem., 273, 25777–25782. - PubMed
-
- Al-Mehdi A.B., et al. (2000) Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med., 6, 100–102. - PubMed
-
- Chang C.C., et al. (2013) Connective tissue growth factor activates pluripotency genes and mesenchymal-epithelial transition in head and neck cancer cells. Cancer Res., 73, 4147–4157. - PubMed
-
- Hsiang C.Y., et al. (2007) Acetaldehyde induces matrix metalloproteinase-9 gene expression via nuclear factor-kappaB and activator protein 1 signaling pathways in human hepatocellular carcinoma cells: association with the invasive potential. Toxicol. Lett., 171, 78–86. - PubMed
-
- Seo J.H., et al. (2004) Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab. Invest., 84, 49–62. - PubMed
-
- Le Bitoux M.A., et al. (2008) Tumor-host interactions: the role of inflammation. Histochem. Cell Biol., 130, 1079–1090. - PubMed
-
- Weaver V.M., et al. (2004) Watch thy neighbor: cancer is a communal affair. J. Cell Sci., 117, 1287–1290. - PubMed
-
- Maguer-Satta V. (2011) The stem cell niche: the black master of cancer. Cancer Stem Cells Theories and Practices, ISBN 978-953-307-225-8.
-
- Ding J., et al. (2009) TNF-alpha induction by nickel compounds is specific through ERKs/AP-1-dependent pathway in human bronchial epithelial cells. Curr. Cancer Drug Targets, 9, 81–90. - PubMed
-
- Li J., et al. (2004) Nickel compounds act through phosphatidylinositol-3-kinase/Akt-dependent, p70(S6k)-independent pathway to induce hypoxia inducible factor transactivation and Cap43 expression in mouse epidermal Cl41 cells. Cancer Res., 64, 94–101. - PubMed
-
- Ding J., et al. (2006) Nickel compounds render anti-apoptotic effect to human bronchial epithelial Beas-2B cells by induction of cyclooxygenase-2 through an IKKbeta/p65-dependent and IKKalpha- and p50-independent pathway. J. Biol. Chem., 281, 39022–39032. - PubMed
-
- Zhang J., et al. (2013) The alteration of miR-222 and its target genes in nickel-induced tumor. Biol. Trace Elem. Res., 152, 267–274. - PubMed
-
- Takahashi A., et al. (2004) Bisphenol A from dental polycarbonate crown upregulates the expression of hTERT. J. Biomed. Mater. Res. B. Appl. Biomater., 71, 214–221. - PubMed
-
- Patel E., et al. (2013) Methylmercury impairs motor function in early development and induces oxidative stress in cerebellar granule cells. Toxicol. Lett., 222, 265–272. - PubMed
-
- Sherwani S.I., et al. (2013) Eicosanoid signaling and vascular dysfunction: methylmercury-induced phospholipase D activation in vascular endothelial cells. Cell Biochem. Biophys., 67, 317–329. - PubMed
-
- Chang X., et al. (2013) Paraquat inhibits cell viability via enhanced oxidative stress and apoptosis in human neural progenitor cells. Chem. Biol. Interact., 206, 248–255. - PubMed
-
- Whiteside T.L. (2006) Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol., 16, 3–15. - PubMed
-
- Whiteside T.L. (2002) Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol., 12, 43–50. - PubMed
-
- Mocellin S., et al. (2004) The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev., 15, 61–76. - PubMed
-
- Zou W. (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer, 5, 263–274. - PubMed
-
- IARC (2013) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Agents classified by the IARC Monographs. International Agency for Research and Cancer, Lyon.
-
- IARC (1982) Monograph on the Evaluation of Carcinogenic Risk to Humans. Some Industrial Chemicals and Dyestuffs. Di(2-ethylhexyl) Phthalate. International Agency for Research and Cancer, Lyon, pp. 257–280.
-
- IARC (2000) Monograph on the Evaluation of Carcinogenic Risk to Humans. Some Industrial Chemicals. Di(2-ethylhexyl) Phthalate. International Agency for Research and Cancer, Lyon, pp. 41–148.
-
- Odermatt A., et al. (2008) Disruption of glucocorticoid and mineralocorticoid receptor-mediated responses by environmental chemicals. CHIMIA Int. J. Chem., 62, 335–339.
-
- Santos P.M., et al. (2009) Insights into yeast adaptive response to the agricultural fungicide mancozeb: a toxicoproteomics approach. Proteomics, 9, 657–670. - PubMed
-
- Kuster M., et al. (2009) Liquid chromatography–tandem mass spectrometric analysis and regulatory issues of polar pesticides in natural and treated waters. J. Chromatogr. A, 1216, 520–529. - PubMed
-
- Çayır A., et al. (2014) Micronuclei, nucleoplasmic bridges, and nuclear buds induced in human lymphocytes by the fungicide signum and its active ingredients (boscalid and pyraclostrobin). Environ. Toxicol, 29, 723–732. - PubMed
-
- Kavlock R., et al. (2012) Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chem. Res. Toxicol., 25, 1287–1302. - PubMed
-
- Martin M.T., et al. (2011) Predictive model of rat reproductive toxicity from ToxCast high throughput screening. Biol. Reprod., 85, 327–339. - PubMed
-
- Metzler M., et al. (2001) Chemistry of natural and anthropogenic endocrine active compounds. In M.Metzler (ed) The Handbook of Environmental Chemistry Vol. 3, Part L. Endocrine Disruptors–Part I. Springer, Berlin Heidelberg, pp. 63–80.
-
- Gatidou G., et al. (2007) Simultaneous determination of the endocrine disrupting compounds nonylphenol, nonylphenol ethoxylates, triclosan and bisphenol A in wastewater and sewage sludge by gas chromatography–mass spectrometry. J. Chromatogr. A, 1138, 32–41. - PubMed
-
- Foran C., et al. (2000) Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar. Environ. Res., 50, 153–156. - PubMed
-
- Ishibashi H., et al. (2004) Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol., 67, 167–179. - PubMed
-
- Boyd G.R., et al. (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ., 311, 135–149. - PubMed
-
- Kolpin D.W., et al. (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol., 36, 1202–1211. - PubMed
-
- Vandhana S., et al. (2013) Biochemical changes accompanying apoptotic cell death in retinoblastoma cancer cells treated with lipogenic enzyme inhibitors. Biochim. Biophys. Acta (BBA)-Molecular and Cell Biology of Lipids, 1831, 1458–1466. - PubMed
-
- Zuckerbraun H.L., et al. (1998) Triclosan, cytotoxicity, mode of action, and induction of apoptosis in human gingival cells in vitro . Eur. J. Oral Sci., 106, 628–636. - PubMed
-
- Terasaka H., et al. (2005) Cytotoxicity and apoptosis-inducing activity of bisphenol A and hydroquinone in HL-60 cells. Anticancer Res., 25, 2241–2247. - PubMed
-
- The Australian National Industrial Chemicals Notification and Assessment Scheme (2006) Diethylhexyl Phthalate (DEHP) Factsheet. CAS: 117-81-7. http://www.nicnas.gov.au/communications/publications/information-sheets/... (7 May 2015, date last accessed).
-
- Hao C., et al. (2012) Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front. Biosci. (Elite edition), 5, 725–733. - PubMed
-
- Moushumi Priya A., et al. (2012) Induction of Apoptosis and cell cycle arrest by Bis (2-ethylhexyl) phthalate produced by Marine Bacillus pumilus MB 40. Chem. Biol. Interact., 195, 133–143. - PubMed
-
- Park M.A., et al. (2012) Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclin-dependent kinase-4 genes. Mol. Med. Rep., 5, 761–766. - PubMed
-
- Kinoshita Y., et al. (2003) Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res., 63, 3546–3555. - PubMed
-
- Laville N., et al. (2006) Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology, 228, 98–108. - PubMed
-
- (2012) A Review of Human Carcinogens—Pharmaceuticals IARC Monographs on the Evaluation of Caricinogenic Risk to Humans. Vol. 100, World health Organization IARC, Geneva, Switzerland.
-
- Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures (2000) U.S. Environmental Protection Agency Report No. EPA/630/R-00/002, Washington, DC.
-
- Dellarco V., et al. (2012) Mode of action: moving toward a more relevant and efficient assessment paradigm. J. Nutr., 142, 2192S–2198S. - PubMed
-
- Meek M.E., et al. (2003) A framework for human relevance analysis of information on carcinogenic modes of action. Crit. Rev. Toxicol., 33, 591–653. - PubMed
-
- Boobis A.R., et al. (2006) IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit. Rev. Toxicol., 36, 781–792. - PubMed
-
- (2012) OECD Guidance Document 116 On The Conduct And Design Of Chronic Toxicity And Carcinogenicity Studies, Supporting Test Guidelines 451, 452 And 453, 2nd Edition, Series on Testing and Assessment, ENV/JM/MONO(2011)47. OECD Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology, Paris, France.
-
- U.S.E.P.A. (2002) Guidance on Cumulative Risk Assessment of Pesticide Chemicals That Have a Common Mechanism of Toxicity. In Office of Pesticide Programs, Washington, D.C. 20460.
-
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) (2013). Scientific opinion on the relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides residues in food. EFSA J., 11, 40.
-
- Driedger A.A., et al. (1971) Demonstration of two types of DNA repair in X-irradiated Micrococcus radiodurans. Can. J. Microbiol., 17, 495–499. - PubMed
-
- Heindorff K., et al. (1983) Genetic toxicology of ethylenediaminetetraacetic acid (EDTA). Mutat. Res., 115, 149–173. - PubMed
-
- Cory-Slechta, D. et al. (2008) Phthalates Cumulative Risk Assessment—The Tasks Ahead. In National Research Council, N.A.o.S., Board on Environmental Science and Technology, Committee on Phthalates Health Risks. National Academy Press, Washington, DC, p. 208.
-
- Bucala R. (1996) MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J., 10, 1607–1613. - PubMed
-
- Bucala R., et al. (2007) Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity, 26, 281–285. - PubMed
-
- Landesmann B., et al. (2013) Adverse outcome pathway-based screening strategies for an animal-free safety assessment of chemicals. Altern. Lab. Anim., 41, 461–471. - PubMed
-
- Malhotra J., et al. (2015) Effect of occupational exposures on lung cancer susceptibility: a study of gene-environment interaction analysis. Cancer Epidemiol. Biomarkers Prev., 24, 570–579. - PubMed
-
- Bisson W.H. (2012) Editorial: computational chemogenomics in drug design and discovery. Curr. Top. Med. Chem., 12, 1867–1868. - PubMed
-
- Weinberg R.A. (2014) Coming full circle-from endless complexity to simplicity and back again. Cell, 157, 267–271. - PubMed
-
- Alberghina L., et al. (2004) Systems biology and the molecular circuits of cancer. Chembiochem, 5, 1322–1333. - PubMed
-
- Porter W.P., et al. (1999) Endocrine, immune, and behavioral effects of aldicarb (carbamate), atrazine (triazine) and nitrate (fertilizer) mixtures at groundwater concentrations. Toxicol. Ind. Health, 15, 133–150. - PubMed
-
- Tarone R.E., et al. (2011) Combating environmental causes of cancer. N. Engl. J. Med., 364, 2266–2267. - PubMed
-
- Willett W.C., et al. (2011) Combating environmental causes of cancer. N. Engl. J. Med., 364, 2266. - PubMed
-
- Richter E.D., et al. (2004) The precautionary principle, epidemiology and the ethics of delay. Int. J. Occup. Med. Environ. Health, 17, 9–16. - PubMed
-
- Pohl H.R., et al. (2010) Chemical risk assessment and uncertainty associated with extrapolation across exposure duration. Regul. Toxicol. Pharmacol., 57, 18–23. - PubMed