Disease-Modifying Drugs and Family Planning in People with Multiple Sclerosis: A Consensus Narrative Review from the Gulf Region
Affiliations
Affiliations
- Department of Medicine, Amiri Hospital, Sharq, Kuwait. alroughani@gmail.com.
- Department of Neurology, Rashid Hospital, Dubai, United Arab Emirates.
- Dubai Medical College, Dubai Health Authority (DHA), Dubai, United Arab Emirates.
- Neurology Unit, Department of Medicine, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman.
- Neuroscience Department, Khoula Hospital, Muscat, Oman.
- Department of Neurology, American Center of Psychiatry and Neurology, Abu Dhabi, United Arab Emirates.
- Neurology and Immunology Medical Affairs Gulf Region, Merck Serono Middle East FZ LTD, Dubai, United Arab Emirates.
- Department of Neurology (Neuroscience Institute), Hamad Medical Corporation, Doha, Qatar.
- Department of Neurology, Ibn Sina Hospital, Kuwait City, Kuwait.
- Neurology Department, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates.
Abstract
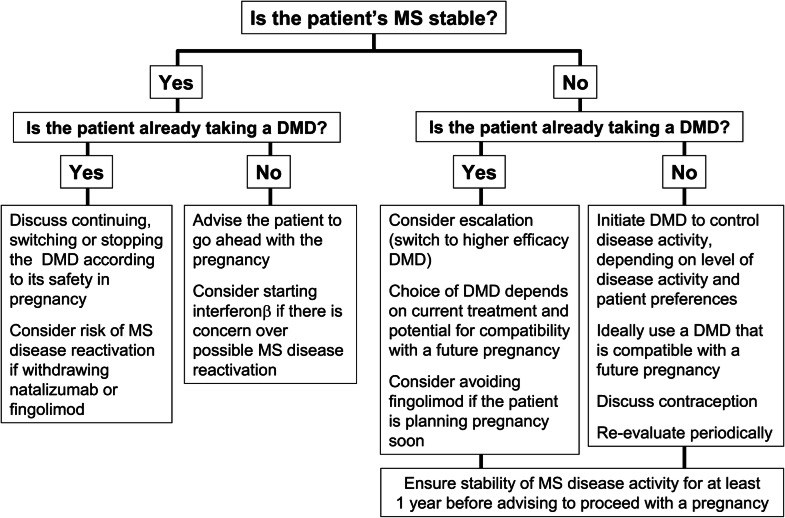
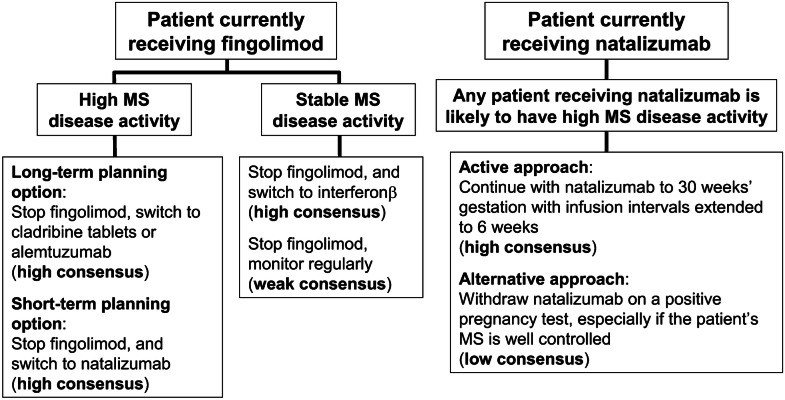
Most disease-modifying drugs (DMDs) are contraindicated in pregnancy. Management of MS is especially challenging for pregnant patients, as withdrawal of DMDs leave the patient at risk of increased disease activity. We, a group of experts in MS care from countries in the Arab Gulf, present our consensus recommendations on the management of MS in these patients. Where possible, a patient planning pregnancy can be switched to a DMD considered safe in this setting. Interferon β now can be used during pregnancy, where there is a clinical need to maintain treatment, in addition to glatiramer acetate. Natalizumab (usually to 30 weeks' gestation for patients with high disease activity at high risk of relapse and disability progression) may also be continued into pregnancy. Cladribine tablets and alemtuzumab have been hypothesised to act as immune reconstitution therapies (IRTs). These drugs provide a period of prolonged freedom from relapses for many patients, but the patient must be prepared to wait for up to 20 months from initiation of therapy before becoming pregnant. If a patient becomes pregnant while taking fingolimod, and requires continued DMD treatment, a switch to interferon β or natalizumab after a variable washout period may be prescribed, depending on the level of disease activity. Women who wish to breastfeed should be encouraged to do so, and interferon β may also be used during breastfeeding. There is a lack of data regarding the safety of using other DMDs during breastfeeding.
Keywords: Breastfeeding; Disease-modifying drugs; Family planning; Multiple sclerosis; Pregnancy.
Figures
Similar articles
Filippini G, Del Giovane C, Clerico M, Beiki O, Mattoscio M, Piazza F, Fredrikson S, Tramacere I, Scalfari A, Salanti G.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2.PMID: 28440858 Free PMC article. Review.
Goldberg LD, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM.J Manag Care Pharm. 2009 Sep;15(7):543-55. doi: 10.18553/jmcp.2009.15.7.543.PMID: 19739877
Inshasi J, Alroughani R, Al-Asmi A, Alkhaboury J, Alsalti A, Boshra A, Canibano B, Deleu D, Ahmed SF, Shatila A, Thakre M.Neurol Ther. 2021 Jun 17:1-17. doi: 10.1007/s40120-021-00260-5. Online ahead of print.PMID: 34155473 Review.
Inshasi J, Alroughani R, Al-Asmi A, Alkhaboury J, Alsalti A, Boshra A, Canibano B, Deleu D, Ahmed SF, Shatila A, Thakre M.Neurol Ther. 2021 Dec;10(2):539-555. doi: 10.1007/s40120-021-00260-5. Epub 2021 Jun 17.PMID: 34138444 Free PMC article. Review.
Cited by
Clavelou P, Castelnovo G, Pourcher V, De Sèze J, Vermersch P, Ben-Amor AF, Savarin C, Defer G.Neurol Ther. 2023 Jun 29. doi: 10.1007/s40120-023-00496-3. Online ahead of print.PMID: 37382841 Review.
Namvar H, Sahraian MA.Oman Med J. 2023 Mar 31;38(2):e479. doi: 10.5001/omj.2023.58. eCollection 2023 Mar.PMID: 37132004 Free PMC article.
De Sèze J, Suchet L, Mekies C, Manchon E, Labauge P, Guennoc AM, Defer G, Clavelou P, Castelnovo G, Bourre B, Bensa-Koscher C, Al Khedr A, Le Mao J, Villemur L, Bouée S, Luciani L, Vermersch P.Neurol Ther. 2023 Apr;12(2):351-369. doi: 10.1007/s40120-022-00430-z. Epub 2022 Dec 24.PMID: 36564664 Free PMC article. Review.
Sánchez-Velasco S, Midaglia L, Vidal-Jordana A, Castillo F, Horno R, Carreras E, Serrano B, Bosch M, Agustí A, Montalban X, Tintoré M.Rev Neurol. 2023 Jan 1;76(1):21-30. doi: 10.33588/rn.7601.2022404.PMID: 36544373 Free PMC article. Review. Spanish.
Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician's Review.
Giovannoni G, Mathews J.Neurol Ther. 2022 Jun;11(2):571-595. doi: 10.1007/s40120-022-00339-7. Epub 2022 Mar 23.PMID: 35318617 Free PMC article. Review.
KMEL References
References
-
- Multiple Sclerosis International Federation. Atlas of MS 2013. Mapping multiple sclerosis around the world. https://www.msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/. Accessed Apr 2020.
-
- Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. - PubMed
-
- Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. 2014;20:520–526. - PubMed
-
- Al Tahan AM, Alsharoqi I, Bohlega SA, et al. Characteristics of multiple sclerosis in the Middle East with special reference to the applicability of international guidelines to the region. Int J Neurosci. 2014;124:635–641. - PubMed
-
- Heydarpour P, Khoshkish S, Abtahi S, Moradi-Lakeh M, Sahraian MA. Multiple sclerosis epidemiology in Middle East and North Africa: a systematic review and meta-analysis. Neuroepidemiology. 2015;44:232–244. - PubMed
-
- Nasr Z, Etemadifar M, Khalili N. Epidemiology of multiple sclerosis in the Middle East: a systematic review and meta analysis. Mult Scler Relat Disord. 2014;3:744.
-
- Habibzadeh F. Editor's Page. Contraception in the Middle East. Lancet Middle East Edition. https://download.thelancet.com/flatcontentassets/pdfs/Sep12_MiddleEastEd.... Accessed Apr 2020.
-
- Roudi-Fahimi F, Abdul Monem A, Ashford L, El-Adawy M. United Nations Population Fund. Women’s need for family planning in Arab countries. https://www.who.int/evidence/resources/policy_briefs/UNFPAPBunmentneed20.... Accessed Feb 2020.
-
- Mendibe Bilbao M, Boyero Durán S, Bárcena Llona J, Rodriguez-Antigüedad A. Multiple sclerosis: pregnancy and women's health issues. Neurologia. 2019;34:259–269. - PubMed
-
- Smeltzer SC. Reproductive decision making in women with multiple sclerosis. J Neurosci Nurs. 2002;34:145–157. - PubMed
-
- Carvalho AT, Veiga A, Morgado J, et al. Multiple sclerosis and motherhood choice: an observational study in Portuguese women patients. Rev Neurol. 2014;59:537–542. - PubMed
-
- Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, Brex PA. UK consensus on pregnancy in multiple sclerosis: 'Association of British Neurologists' guidelines. Pract Neurol. 2019;19:106–114. - PubMed
-
- American Academy of Neurology. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. https://www.aan.com/Guidelines/home/GuidelineDetail/898. Accessed Apr 2020.
-
- Dyment DA, Sadovnick AD, Ebers GC. Genetics of multiple sclerosis. Hum Mol Genet. 1997;6:1693–1698. - PubMed
-
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. - PubMed
-
- Hellwig K, Gold R. Family planning and multiple sclerosis. Akt Neurol. 2010;37:292–303.
-
- Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. - PubMed
-
- Amato MP, Bertolotto A, Brunelli R, et al. Management of pregnancy-related issues in multiple sclerosis patients: the need for an interdisciplinary approach (published correction appears in Neurol Sci. 2017 Dec 26) Neurol Sci. 2017;38:1849–1858. - PubMed
-
- Alroughani R, Akhtar S, Zeineddine M, et al. Risk of relapses during pregnancy among multiple sclerosis patients. Mult Scler Relat Disord. 2019;34:9–13. - PubMed
-
- Herbstritt S, Langer-Gould A, et al. Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler. 2016;22:810–816. - PubMed
-
- Hellwig K, Rog D, McGuigan C, Chen K, Parks B, Jones CC. An international registry tracking pregnancy outcomes in women treated with dimethyl fumarate. Abstract (P1147) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278349/.... Accessed Apr 2020.
-
- Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-β exposure in multiple sclerosis. Neurology. 2010;75:1794–1802. - PubMed
-
- Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis—a prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. 2016;22:801–809. - PubMed
-
- Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011;17:423–430. - PubMed
-
- Hellwig K, Geissbuehler Y, Sabidó M, et al. Pregnancy and infant outcomes with interferon beta: data from the European Interferon Beta Pregnancy Registry and Population Based Registries in Finland and Sweden. Abstract (A-0950-0000-02658) and poster at the ECTRIMS 2018 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2018/ectrims-2018/2281.... Accessed Apr 2020.
-
- Vukusic S, Hellwig K, Truffinet P, et al. Pregnancy outcomes in female partners of male patients treated with teriflunomide or leflunomide (an in vivo precursor of teriflunomide). Abstract (P1146) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278348/.... Accessed Apr 2020.
-
- Lebrun-Frenay C, Rog D, Benamor M, Jurgensen S, Truffinet P, Ghezzi A. Teriflunomide (Aubagio®) International Pregnancy Registry: Enrollment Update (P4.371). Neurology 2018;90 (15 Supplement). Abstract available at https://n.neurology.org/content/90/15_Supplement/P4.371. Accessed Apr 2020.
-
- Truffinet P, Afsar S, Davenport L, Purvis A, Poole EM, Henson LJ. Pregnancy outcomes in patients treated with leflunomide, an in vivo precursor of the multiple sclerosis drug teriflunomide. Abstract (P1148) at the ECTRIMS 2019 congress, available at https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278350/.... Accessed Apr 2020.
-
- Achiron A, Chambers C, Fox EJ, et al. Pregnancy outcomes in patients with active RRMS who received alemtuzumab in the clinical development program. Abstract (P1120) and poster at the ECTRIMS 2015 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2015/31st/116041/anat..... Accessed Apr 2020.
-
- Rog D, Jiwon O, Chambers C, et al. Pregnancy outcomes in patients with RRMS treated with alemtuzumab from the clinical development program. Abstract (P749) and poster at the ECTRIMS 2017 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS20.... Accessed Apr 2020.
-
- Galazka A, Nolting A, Cook S, et al. Pregnancy outcomes during the clinical development programme of cladribine in multiple sclerosis (MS): an integrated analysis of safety for all exposed patients. Abstract (P1874) at the ECTRIMS 2017 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS20.... Accessed Apr 2020.
-
- Lopez Leon S, Geissbuehler Y, Moore A, et al. Effect of fingolimod on pregnancy outcomes in patients with multiple sclerosis. Abstract (P411) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278772/.... Accessed Apr 2020.
-
- European Medicines Agency. Updated restrictions for Gilenya: multiple sclerosis medicine not to be used in pregnancy. Press release 26/07/2019. https://www.ema.europa.eu/en/news/updated-restrictions-gilenya-multiple-.... Accessed Apr 2020.
-
- Ebrahimi N, Herbstritt S, Gold R, Amezcua L, Koren G, Hellwig K. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler. 2015;21:198–205. - PubMed
-
- Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology. 2018;90:e823–e831. - PubMed
-
- Triplett JD, Vijayan S, Rajanayagam S, Tuch P, Kermode AG. Pregnancy outcomes amongst multiple sclerosis females with third trimester natalizumab use. Mult Scler Relat Disord. 2020;40:101961. - PubMed
-
- Oreja-Guevara C, Wray S, Buffels R, Zecevik D, Vukusik S. Pregnancy outcomes in patients treated with ocrelizumab. Abstract (P780) and poster at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279140/.... Accessed Apr 2020.
-
- Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16:56–62. - PubMed
-
- European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada (November 2019). https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Apr 2020.
-
- Haghikia A, Langer-Gould A, Rellensmann G, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. 2014;71:891–895. - PubMed
-
- Miller AE. Teriflunomide: a once-daily oral medication for the treatment of relapsing forms of multiple sclerosis. Clin Ther. 2015;37:2366–2380. - PubMed
-
- Genzyme Corporation. Elimination Procedure—AUBAGIO® (teriflunomide). https://www.aubagiohcp.com/content/pdf/drug_elimination_guide.pdf. Accessed Apr 2020.
-
- Portaccio E, Moiola L, Martinelli V, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology. 2018;90:e832–e839. - PubMed
-
- Sepúlveda M, Montejo C, Llufriu S, et al. Rebound of multiple sclerosis activity after fingolimod withdrawal due to planning pregnancy: analysis of predisposing factors. Mult Scler Relat Disord. 2019;38:101483. - PubMed
-
- Hemat S. Disease activity during pregnancy after fingolimod withdrawal due to planning a pregnancy in women with multiple sclerosis. Abstract (207) at the ECTRIMS 2018 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2018/ectrims-2018/2319.... Accessed Apr 2020.
-
- Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology. 2018;90:e840–e846. - PubMed
-
- Ladeira F, Braz L, Salgado P, Vaz S. A multicenter, non-interventional study to evaluate the disease activity in multiple sclerosis after withdrawal of Natalizumab in Portugal. Clin Neurol Neurosurg. 2019;184:105390. - PubMed
-
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–392. - PubMed
-
- KIOVIG 100 mg/L solution for infusion. European Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/9198. Accessed Apr 2020.
-
- Armon C, Baquis GD, Howard GF III, et al. Neurologic Disease and Pregnancy. Multiple Sclerosis. Updated: Aug 20, 2019. https://emedicine.medscape.com/article/1149405-overview#a7. Accessed Apr 2020.