Economic burden of multiple sclerosis on Kuwait health care system
Affiliations
Affiliations
- Department of Pharmacy Practice, School of Pharmacy, Kuwait University, Jabriya, Kuwait.
- Department of Neurology, Ibn Sina Hospital, Sabah Medical Area, Kuwait.
- Department of Neurology and Psychiatry, Minia University, Minia, Egypt.
- Department of Medicine, Faculty of Medicine, Kuwait University, Jabriya, Kuwait.
- Division of Neurology, Department of Medicine, Amiri Hospital, Sharq, Kuwait.
Abstract
Background: Multiple Sclerosis (MS) is a chronic neurological disease with heavy economic and social burdens resulting in significant disability.
Objective: This study aims to (1) measure the cost of health resources utilization by MS patients and (2) to examine the difference in utilization and its attributed costs amongst patients who may have a different course of MS and expanded disability status scale (EDSS) scores.
Methods: A cross-sectional study using Kuwait National MS registry was conducted to estimate the costs of utilization of resources from 2011 to 2015.
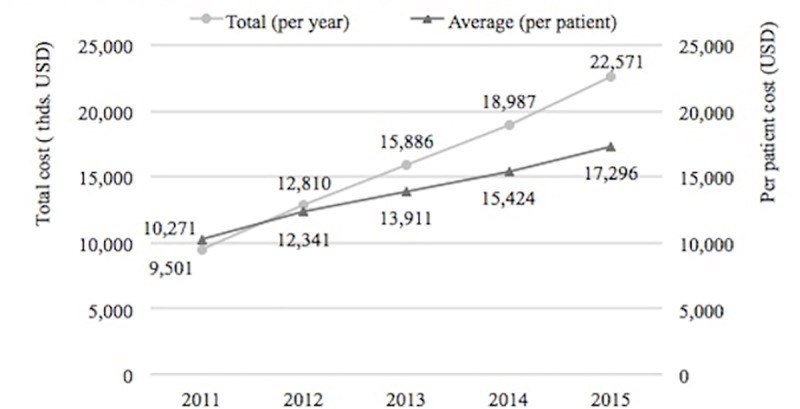
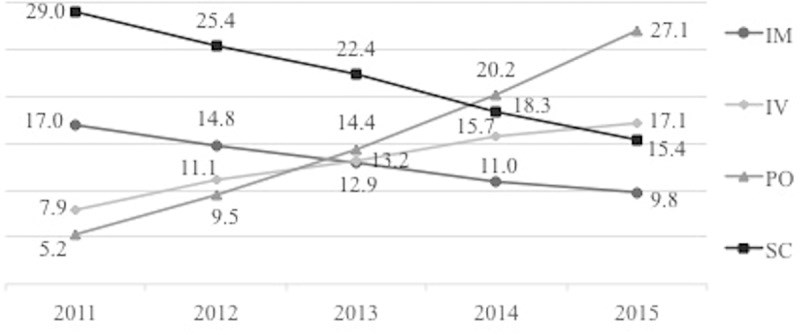
Results: Between the period 2011-2015, 1344 MS patients were included in the registry. The average annual cost per MS patient has increased from $10,271 in 2011 to $17,296 in 2015. Utilization of disease-modifying therapies (DMTs) was the main driver of costs reaching 89.9% in 2015. Throughout the five-year period, the occurrence of relapses decreased from 21.8% to 12.2% (p <0.0001). During this same period, ambulatory relapse treatment increased by 5.8% while hospitalizations decreased by 2.6%. Patients with a moderate EDSS score (3.5-6) had the highest average cost (p<0.0001) compared to mild and severe EDSS scores.
Conclusions: Multiple sclerosis has been a significant economic burden on the Kuwait healthcare system. DMTs are the main driver of cost.
Conflict of interest statement
Dr Alroughani received honoraria as a speaker and for serving in scientific advisory board from Bayer, Biogen, Novartis, Sanofi-Genzyme, Roche and Merck. Drs Alowayesh, Ahmed, and Al-Hashel have nothing to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH.J Popul Ther Clin Pharmacol. 2012;19(1):e11-25. Epub 2012 Jan 10.PMID: 22247419
Cost analysis of multiple sclerosis in Brazil: a cross-sectional multicenter study.
da Silva NL, Takemoto MLS, Damasceno A, Fragoso YD, Finkelsztejn A, Becker J, Gonçalves MVM, Tilbery C, de Oliveira EML, Callegaro D, Boulos FC.BMC Health Serv Res. 2016 Mar 24;16:102. doi: 10.1186/s12913-016-1352-3.PMID: 27009599 Free PMC article.
Ruutiainen J, Viita AM, Hahl J, Sundell J, Nissinen H.J Med Econ. 2016;19(1):21-33. doi: 10.3111/13696998.2015.1086362. Epub 2015 Sep 11.PMID: 26360615
Mitoxantrone: a review of its use in multiple sclerosis.
Scott LJ, Figgitt DP.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010.PMID: 15089110 Review.
There is much to be learnt about the costs of multiple sclerosis in Latin America.
Romano M, Machnicki G, Rojas JI, Frider N, Correale J.Arq Neuropsiquiatr. 2013 Aug;71(8):549-55. doi: 10.1590/0004-282X20130082.PMID: 23982015 Review.
Cited by
Economic Burden of Multiple Sclerosis Drugs in Iran during 2011-2019.
Asadollahi M, Darvishi A, Azimi A, Annabi M, Jafariazar Z, Heshmat R.Iran J Public Health. 2023 Feb;52(2):407-419. doi: 10.18502/ijph.v52i2.11894.PMID: 37089141 Free PMC article.
Estimation the medical cost of multiple sclerosis in Iran; 2019-2020.
Asadollahi M, Darvishi A, Azimi A, Annabi M, Jafariazar Z, Heshmat R.BMC Health Serv Res. 2022 Feb 2;22(1):137. doi: 10.1186/s12913-022-07551-z.PMID: 35109835 Free PMC article.
Economic burden of multiple sclerosis: a cross-sectional study in Iran.
Rezaee M, Keshavarz K, Izadi S, Jafari A, Ravangard R.Health Econ Rev. 2022 Jan 3;12(1):2. doi: 10.1186/s13561-021-00350-y.PMID: 34981265 Free PMC article.
Alroughani R, Van Wijmeersch B, Al Khaboori J, Alsharoqi IA, Ahmed SF, Hassan A, Inshasi J, Krieger DW, Shakra M, Shatila AO, Szolics M, Khallaf M, Ezzat A.Ther Adv Neurol Disord. 2020 Sep 16;13:1756286420954119. doi: 10.1177/1756286420954119. eCollection 2020.PMID: 32973927 Free PMC article. Review.
KMEL References
References
-
- Kobelt G, Pugliatti M. Cost of multiple sclerosis in Europe. Eur J Neurol 2005;12(Supp. 1):S63–S67.
-
- Pohar SL, Jones CA, Warren S, Turpin KV, Warren K. Health status and health care utilization of multiple sclerosis in Canada. Can J Neurol Sci 2007;34(2):167–174. - PubMed
-
- Pope GC, Urato CJ, Kulas ED, Kronick R, Gilmer T. Prevalence, expenditures, utilization, and payment for persons with MS in insured populations (provisional record). Neurology 2002;58:37–43. - PubMed
-
- Gottberg K, Einarsson U, Fredrikson S, von Koch L, Holmqvist LW. Multiple sclerosis in Stockholm County: a pilot study of utilization of health-care resources, patient satisfaction with care and impact on family caregivers. Acta Neurol Scand 2002;106(5):241–247. - PubMed
-
- Beckerman H, van Zee IE, de Groot V, van den Bos GA, Lankhorst GJ, Dekker J. Utilization of health care by patients with multiple sclerosis is based on professional and patient-defined health needs. Mult Scler 2008;00:1–11. - PubMed
-
- Stolp-Smith KA, Atkinson EJ, Campion ME, O'Brien PC, Rodriguez M. Health care utilization in multiple sclerosis: a population-based study in Olmsted County, MN. Neurology 1998;50(6):1594–1600. - PubMed
-
- Kanavos P, Tinelli M, Efthymiadou O, Visintin E, Grimaccia F, Mossman J. Towards better outcomes in multiple sclerosis by addressing policy change:The International MultiPlE Sclerosis Study (IMPrESS). London School of Economics; UK: 2016
-
- Lublin FD and Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911. - PubMed
-
- Trisolini M, Honeycutt A, Wiener J, Lesesne S. Global Economic Impact of Multiple Sclerosis: A Literature Review. Multiple Sclerosis International Federation (UK) and RTI International (USA) 2010.
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33(11):1444–1452. - PubMed
-
- Dunn OJ. Multiple comparisons using rank sums. Technometrics 1963; 6:241–252.
-
- Hurvich CM, Tsai CL. Bias of the corrected AIC criterion for under fitted regression and time series models. Biomelrika 1991;78:3:499–509.
-
- Jones E, Pike J, Marshall T, Ye X. Quantifying the relationship between increased disability and health care resource utilization, quality of life, work productivity, health care costs in patients with multiple sclerosis in the US. BMC Health Services Research 2016;16:294–304 10.1186/s12913-016-1532-1 - DOI - PMC - PubMed
-
- Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017;16(4):271–281. 10.1016/S1474-4422(17)30007-8 - DOI - PubMed
-
- Fox RJ, Chan A, Zhang A, Xiao J, Levison D, Lewin JB, et al. Comparative effectiveness using a matching-adjusted indirect comparison between delayed-release dimethyl fumarate and fingolimod for the treatment of multiple sclerosis. Curr Med Res Opin 2017;33(2):175–183. 10.1080/03007995.2016.1248380 - DOI - PubMed
-
- Bergvall N, Petrilla AA, Karkare SU, Lahoz R, Agashivala N, Pradhan A, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ 2014;17(10):696–707. 10.3111/13696998.2014.940422 - DOI - PubMed