A systematic review of cases of CNS demyelination following COVID-19 vaccination
Affiliations
Affiliations
- Department of Neurology, Ibn Sina Hospital, Kuwait. Electronic address: dr.ismail.ibrahim2012@gmail.com.
- Department of Neurology and Psychiatry, University of Alexandria, Alexandria, Egypt.
Abstract
Background: Since the emergency use approval of different types of COVID-19 vaccines, several safety concerns have been raised regarding its early and delayed impact on the nervous system.
Objective: This study aims to systematically review the reported cases of CNS demyelination in association with COVID-19 vaccination, which has not been performed, to our knowledge.
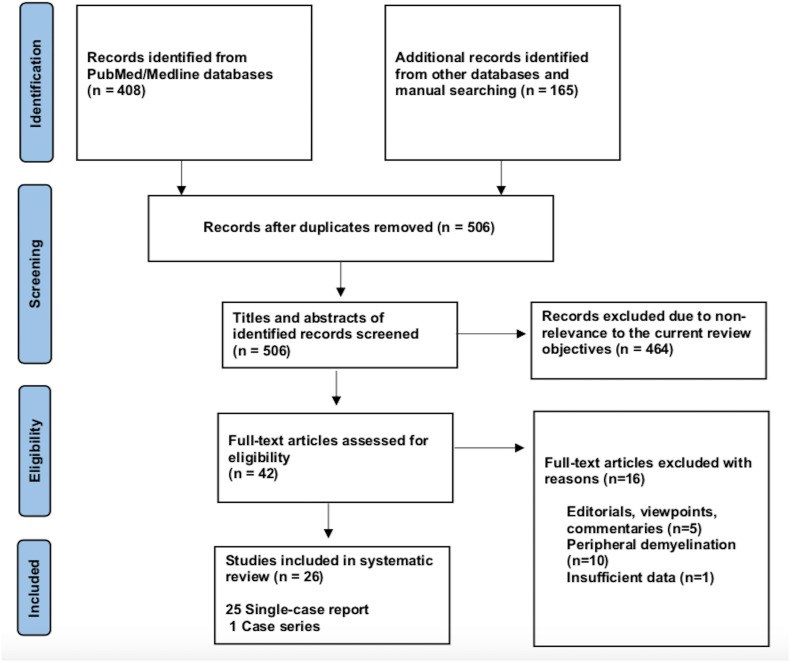
Methods: A systematic review was performed by screening published articles and preprints of cases of CNS demyelination in association with COVID-19 vaccines in PubMed, SCOPUS, EMBASE, Google Scholar, Ovid and medRxiv databases, until September 30, 2021. This study followed PRISMA guidelines. Descriptive findings of reported cases were reviewed and stratified by demographic and clinical findings, diagnostic work-up, management, and overall outcome.
Results: A total of 32 cases were identified, with female predominance (68.8%) and median age of 44 years. Eleven cases were reported after Pfizer vaccine, 8 following AstraZeneca vaccine, 6 following Moderna, 5 following Sinovac/ Sinopharm vaccines, and one following each of Sputnik and Johnson&Johnson vaccines. The majority of cases (71.8%) occurred after the first dose of the vaccine, with neurological symptoms manifesting after a median of 9 days. The most common reported presentations were transverse myelitis (12/32) and MS-like pictures (first diagnosis or a relapse) in another 12/32 cases, followed by ADEM- like (5/32), and NMOSD- like (3/32) presentations. History of a previous immune-mediated disease was reported in 17/32 (53.1%) cases. The mRNA-based vaccines resulted in the greatest number of demyelinating syndromes (17/32), followed by viral vector vaccines (10/32), and inactivated vaccines (5/32). Most MS-like episodes (9/12) were triggered by mRNA-based vaccines, while TM occurred following both viral vector and mRNA-based vaccines. Management included high dose methylprednisolone, PLEX, IVIg, or a combination of those, with a favorable outcome in the majority of case; marked/complete improvement (25/32) or stabilized/ partial recovery in the remaining cases.
Conclusion: This systematic review identified few cases of CNS demyelination following all types of approved COVID-19 vaccines so far. Clinical presentation was heterogenous, mainly following the first dose, however, half of the reported cases had a history of immune-mediated disease. Favorable outcome was observed in most cases. We suggest long-term post-marketing surveillance for these cases, to assess for causality, and ensure the safety of COVID-19 vaccines.
Keywords: COVID-19; Demyelinating disease; Multiple sclerosis; SARS-CoV-2; Transverse myelitis; Vaccine.
Figures
Similar articles
CNS demyelinating disease following inactivated or viral vector SARS-CoV-2 vaccines: A case series.
Ebrahimi N, Mazdak M, Shaygannejad V, Mirmosayyeb O.Vaccine. 2023 Jan 27;41(5):1003-1008. doi: 10.1016/j.vaccine.2023.01.003. Epub 2023 Jan 6.PMID: 36635139 Free PMC article.
Association of CNS demyelination and COVID-19 infection: an updated systematic review.
Ismail II, Salama S.J Neurol. 2022 Feb;269(2):541-576. doi: 10.1007/s00415-021-10752-x. Epub 2021 Aug 12.PMID: 34386902 Free PMC article. Review.
Ciampi E, Uribe-San-Martin R, Soler B, García L, Guzman J, Pelayo C, Jürgensen L, Guzman I, Vera F, Galleguillos L, Cárcamo C.Mult Scler Relat Disord. 2022 Mar;59:103690. doi: 10.1016/j.msard.2022.103690. Epub 2022 Feb 13.PMID: 35182880 Free PMC article.
Monte G, Papetti L, Ferilli MAN, Ursitti F, Moavero R, Sforza G, Panella E, Tarantino S, Checchi MP, Vigevano F, Palma P, Valeriani M.Front Immunol. 2023 Jan 25;14:1106472. doi: 10.3389/fimmu.2023.1106472. eCollection 2023.PMID: 36761740 Free PMC article.
Ostovan VR, Sahraian MA, Karazhian N, Rostamihosseinkhani M, Salimi M, Marbooti H.Mult Scler Relat Disord. 2022 Oct;66:104032. doi: 10.1016/j.msard.2022.104032. Epub 2022 Jul 6.PMID: 35858499 Free PMC article. Review.
Cited by
Tavazzi E, Pichiecchio A, Colombo E, Rigoni E, Asteggiano C, Vegezzi E, Masi F, Greco G, Bastianello S, Bergamaschi R.Viruses. 2023 Jul 18;15(7):1569. doi: 10.3390/v15071569.PMID: 37515255 Free PMC article. Review.
New Onset Autoimmune Diseases after the Sputnik Vaccine.
Vera-Lastra O, Mora G, Lucas-Hernández A, Ordinola-Navarro A, Rodríguez-Chávez E, Peralta-Amaro AL, Medina G, Cruz-Dominguez MP, Jara LJ, Shoenfeld Y.Biomedicines. 2023 Jul 4;11(7):1898. doi: 10.3390/biomedicines11071898.PMID: 37509537 Free PMC article.
Harel T, Gorman EF, Wallin MT.Front Neurol. 2023 Jun 22;14:1099758. doi: 10.3389/fneur.2023.1099758. eCollection 2023.PMID: 37426444 Free PMC article. Review.
Cohen M, Thomel-Rocchi O, Siva A, Okuda DT, Karabudak R, Efendi H, Terzi M, Carra-Dalliere C, Durand-Dubief F, Thouvenot E, Ciron J, Zephir H, Bourre B, Casez O, De Seze J, Moreau T, Neau JP, Pelletier D, Kantarci O, Tutuncu M, Derache N, Bensa C, Louapre C, Benoit J, Landes-Chateau C, Lebrun-Frenay C.Mult Scler. 2023 Jun 15:13524585231179669. doi: 10.1177/13524585231179669. Online ahead of print.PMID: 37322880 Free PMC article.
Central nervous system demyelinating diseases: glial cells at the hub of pathology.
Coutinho Costa VG, Araújo SE, Alves-Leon SV, Gomes FCA.Front Immunol. 2023 May 16;14:1135540. doi: 10.3389/fimmu.2023.1135540. eCollection 2023.PMID: 37261349 Free PMC article. Review.
KMEL References
References
-
- Agmon-Levin N., Kivity S., Szyper-Kravitz M., Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009 Nov;18(13):1198–1204. - PubMed
-
- Aharon-Maor A., Shoenfeld Y. The good, the bad and the ugly of vaccination. Isr. Med. Assoc. J. 2000 Mar;2(3):225–227. - PubMed
-
- Alshararni Ali. Acute transverse Myelitis associated with COVID-19 vaccine: a case report. IJRPS. 2021 Jul 26;12(3):2083–2087.
-
- Angum F., Khan T., Kaler J., Siddiqui L., Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020 May 13;12(5):e8094. doi: 10.7759/cureus.8094. https://www.cureus.com/articles/31952-the-prevalence-of-autoimmune-disor... [Internet]. [cited 2021 Sep 2]; Available from: (PMID: 32542149; PMCID: PMC7292717) - DOI - PMC - PubMed
-
- CDC . US Department of Health and Human Services, CDC; Atlanata, GA: 2021. Vaccine Adverse Event Reporting System (VARES) Accessed August 15, 2021. 2021.
-
- von Csefalvay C. VAERS data reveals no increased risk of neuroautoimmune adverse events from COVID-19 vaccines [Internet] Inf. Dis. (except HIV/AIDS) 2021 Jun doi: 10.1101/2021.06.13.21258851. [cited 2021 Sep 2]. Available from: - DOI
-
- De Martino M., Chiappini E., Galli L. Vaccines and autoimmunity. Int. J. Immunopathol. Pharmacol. 2013 Apr;26(2):283–290. - PubMed
-
- Erdem N.Ş., Demirci S., Özel T., Mamadova K., Karaali K., Çelik H.T., et al. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy Sz. 2021;74(7–8):273–276. - PubMed
-
- Farez M.F., Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J. Neurol. 2011 Jul;258(7):1197–1206. - PubMed
-
- Fitzsimmons W., Nance C.S. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. SSRN J. 2021 https://www.ssrn.com/abstract=3841558 [Internet]. [cited 2021 Sep 2]; Available from:
-
- García-Grimshaw M., Ceballos-Liceaga S.E., Hernández-Vanegas L.E., Núñez I., Hernández-Valdivia N., Carrillo-García D.A., et al. Systemic and Neurologic Adverse Events Among 704,003 First-Dose Recipients of the Pfizer-Biontech (BNT162b2) mRNA COVID-19 Vaccine in Mexico. SSRN J. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. https://www.ssrn.com/abstract=3816489 [Internet]. [cited 2021 Sep 2]; Available from: - DOI - PMC - PubMed
-
- Helmchen C., Buttler G.M., Markewitz R., Hummel K., Wiendl H., Boppel T. Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2. J. Neurol. 2021 Jun 15 doi: 10.1007/s00415-021-10647-x. [Internet]. [cited 2021 Sep 2]; Available from: - DOI - PMC - PubMed
-
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014 Mar;13(3):215–224. - PubMed
-
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014 Mar;13(3):215–224. - PubMed
-
- Mahase E. Covid-19: Oxford researchers halt vaccine trial while adverse reaction is investigated. BMJ. 2020 Sep;9:m3525. - PubMed
-
- Mailand M.T., Frederiksen J.L. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017 Jun;264(6):1035–1050. - PubMed
-
- Mathew T., John S.K. COVID-19 vaccine (ChAdOx1 nCoV-19 Corona virus vaccine (Recombinant)) – COVISHIELD related MS relapse. Neuroimmunol. Reports. 2021 Dec;1:100006.
-
- McLean P., Trefts L. Transverse myelitis 48 hours after the administration of an mRNA COVID 19 vaccine. Neuroimmunol. Reports. 2021 Dec;1:100019.
-
- Raknuzzaman M., Jannaty T., Hossain M.B., Saha B., Dey S.K., Shahidullah M. Post Covid19 vaccination acute disseminated encephalomyelitis: a case report in Bangladesh. Int. J. Med. Sci. Clin. Res. Stud. 2021;1(03):31–36.
-
- Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: a case report. Mult. Scler. 2021 Sep;30 135245852110402. - PubMed
-
- Salemi S., D’Amelio R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 2010 Jun;29(3):247–269. - PubMed
-
- Somers E.C., Thomas S.L., Smeeth L., Hall A.J. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am. J. Epidemiol. 2009 Jan 6;169(6):749–755. - PubMed
-
- Tahir N., Koorapati G., Prasad S., Jeelani H.M., Sherchan R., Shrestha J., et al. SARS-CoV-2 vaccination-induced transverse Myelitis. Cureus. 2021 Jul 25;13(7):e16624. https://www.cureus.com/articles/63667-sars-cov-2-vaccination-induced-tra... [Internet]. [cited 2021 Sep 2]; Available from: - PMC - PubMed
-
- Vera-Lastra O., Medina G., Cruz-Dominguez M.D.P., Jara L.J., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert. Rev. Clin. Immunol. 2013 Apr;9(4):361–373. - PubMed
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan;397(10269):99–111. - PMC - PubMed
-
- World Health Organisation WHO Coronavirus Disease (COVID-19) Dashboard. [Internet] 2021. https://covid19.who.int/ [cited 2021 Sep 1]. Available from:
-
- Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003 Nov;362(9396):1659–1666. - PubMed