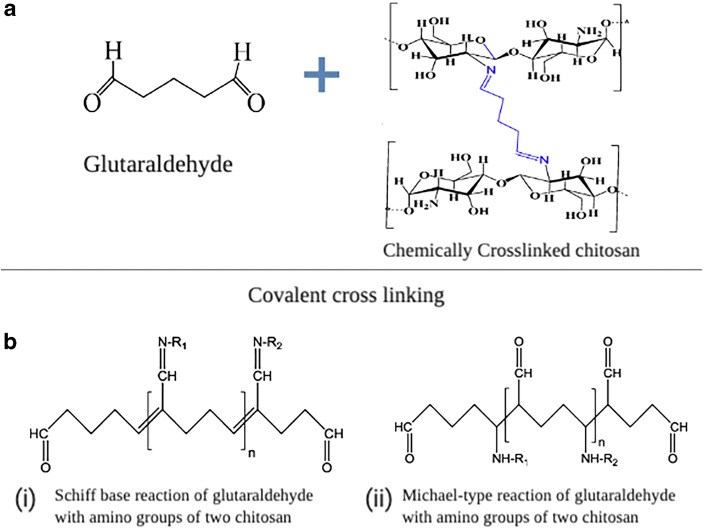
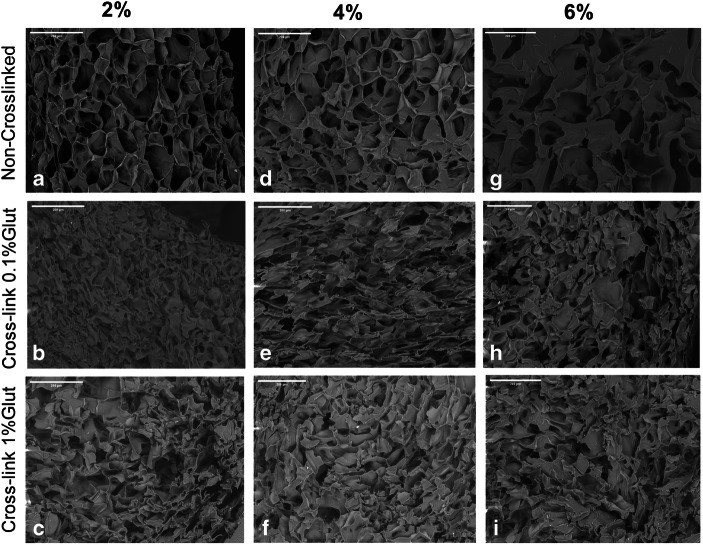
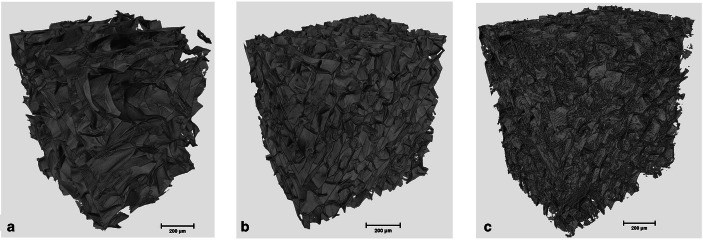
The Effect of Cross-linking Efficiency of Drug-Loaded Novel Freeze Gelated Chitosan Templates for Periodontal Tissue Regeneration
Affiliations
Affiliations
- Department of Biomaterials, Institute of Clinical Dentistry, University of Oslo, Oslo, Norway. ssbqasim@odont.uio.no.
- Department of Bioclinical Sciences, Faculty of Dentistry, Kuwait University, PO-Box 24923, 11310, Safat, Kuwait. ssbqasim@odont.uio.no.
- Department of Biomaterials, Institute of Clinical Dentistry, University of Oslo, Oslo, Norway.
- UWA Dental School, University of Western Australia, 17 Monash Avenue, Nedlands, WA, 6009, Australia.
- Clinical Dentistry Division, Restorative Division, School of Dentistry, International Medical University Kuala Lumpur, 126, Jalan Jalil Perkasa 19, Bukit Jalil 57000, Wilayah Persekutuan, Kuala Lumpur, Malaysia.
Abstract
Innovative strategies for periodontal regeneration have been the focus of research clusters across the globe for decades. In order to overcome the drawbacks of currently available options, investigators have suggested a novel concept of functionally graded membrane (FGM) templates with different structural and morphological gradients. Chitosan (CH) has been used in the past for similar purpose. However, the composite formulation of composite and tetracycline when cross-linked with glutaraldehyde have received little attention. Therefore, the purpose of the study was to investigate the drug loading and release characteristics of novel freeze gelated chitosan templates at different percentages of glutaraldehyde. These were cross-linked with 0.1 and 1% glutaraldehyde and loaded with doxycycline hyclate. The electron micrographs depicted porous morphology of neat templates. After cross-linking, these templates showed compressed ultrastructures. Computerized tomography analysis showed that the templates had 88 to 92% porosity with average pore diameter decreased from 78 to 44.9 μm with increasing concentration. Fourier transform infrared spectroscopy showed alterations in the glycosidic segment of chitosan fingerprint region which after drug loading showed a dominant doxycycline spectral composite profile. Interestingly, swelling profile was not affected by cross-linking either at 0.1 and 1% glutaraldehyde and template showed a swelling ratio of 80%, which gained equilibrium after 15 min. The drug release pattern also showed a 40 μg/mL of release after 24 h. These doxycycline-loaded templates show their tendency to be used in a functionally graded membrane facing the defect site.
Keywords: chitosan; doxycycline hyclate; functionally graded; glutaraldehyde; periodontitis.
Figures
Similar articles
Freeze gelated porous membranes for periodontal tissue regeneration.
Qasim SB, Delaine-Smith RM, Fey T, Rawlinson A, Rehman IU.Acta Biomater. 2015 Sep;23:317-328. doi: 10.1016/j.actbio.2015.05.001. Epub 2015 May 9.PMID: 25968357
Biocompatibility testing of chitosan hydrogels.
Cheburu CN, Stoica B, Neamţu A, Vasile C.Rev Med Chir Soc Med Nat Iasi. 2011 Jul-Sep;115(3):864-70.PMID: 22046800
Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration.
Varoni EM, Vijayakumar S, Canciani E, Cochis A, De Nardo L, Lodi G, Rimondini L, Cerruti M.J Dent Res. 2018 Mar;97(3):303-311. doi: 10.1177/0022034517736255. Epub 2017 Oct 18.PMID: 29045803
Soares DG, Bordini EAF, Cassiano FB, Bronze-Uhle ES, Pacheco LE, Zabeo G, Hebling J, Lisboa-Filho PN, Bottino MC, de Souza Costa CA.J Biomed Mater Res B Appl Biomater. 2020 Aug;108(6):2546-2559. doi: 10.1002/jbm.b.34586. Epub 2020 Feb 15.PMID: 32061059
Drug-Loaded Chitosan Scaffolds for Periodontal Tissue Regeneration.
Atia GAN, Shalaby HK, Zehravi M, Ghobashy MM, Attia HAN, Ahmad Z, Khan FS, Dey A, Mukerjee N, Alexiou A, Rahman MH, Klepacka J, Najda A.Polymers (Basel). 2022 Aug 5;14(15):3192. doi: 10.3390/polym14153192.PMID: 35956708 Free PMC article. Review.
Cited by
Amato M, Santonocito S, Polizzi A, Tartaglia GM, Ronsivalle V, Viglianisi G, Grippaudo C, Isola G.Pharmaceutics. 2023 Apr 21;15(4):1312. doi: 10.3390/pharmaceutics15041312.PMID: 37111796 Free PMC article. Review.
Antibiotic-Loaded Polymeric Barrier Membranes for Guided Bone/Tissue Regeneration: A Mini-Review.
Toledano-Osorio M, Vallecillo C, Vallecillo-Rivas M, Manzano-Moreno FJ, Osorio R.Polymers (Basel). 2022 Feb 21;14(4):840. doi: 10.3390/polym14040840.PMID: 35215754 Free PMC article. Review.
Periodontitis and Systemic Disorder-An Overview of Relation and Novel Treatment Modalities.
Jain P, Hassan N, Khatoon K, Mirza MA, Naseef PP, Kuruniyan MS, Iqbal Z.Pharmaceutics. 2021 Jul 30;13(8):1175. doi: 10.3390/pharmaceutics13081175.PMID: 34452136 Free PMC article. Review.
Polysaccharide-Based Drug Delivery Systems for the Treatment of Periodontitis.
Baranov N, Popa M, Atanase LI, Ichim DL.Molecules. 2021 May 6;26(9):2735. doi: 10.3390/molecules26092735.PMID: 34066568 Free PMC article. Review.
KMEL References
References
-
- Stavropoulos A, Karring T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss ®) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J Clin Periodontol. 2010;37:200–210. doi: 10.1111/j.1600-051X.2009.01520.x. - DOI - PubMed
-
- Marco B, Eliseu M, Maria TA, Divya P. Tetracycline-incorporated nanofibrous coating on titanium to prevent early implant infection and enhance cell response. Front Bioeng Biotechnol. 2016;4. 10.3389/conf.FBIOE.2016.01.00761.
-
- Jin RM, Sultana N, Baba S, Hamdan S, Ismail AF. Porous PCL/chitosan and nHA/PCL/chitosan scaffolds for tissue engineering applications: fabrication and evaluation. J Nanomater. 2015;2015:1–8. doi: 10.1155/2015/357372. - DOI
-
- Giri TK, Thakur A, Alexander A, Ajazuddin, Badwaik H, Tripathi DK. Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications. Acta Pharm Sin B. 2012;2:439–449. doi: 10.1016/j.apsb.2012.07.004. - DOI
-
- Chen M-C, Mi F-L, Liao Z-X, Sung H-W. Chitosan: its applications in drug-eluting devices. In: Jayakumar R, Prabaharan M, Muzzarelli RAA, editors. Chitosan Biomater. I, vol. 243, Springer Berlin Heidelberg; 2011, p. 185–230. 10.1007/12_2011_116.
-
- Mi F-LL, Kuan C-YY, Shyu S-SS, Lee S-TT, Chang S-FF. Study of gelation kinetics and chain-relaxation properties of glutaraldehyde-cross-linked chitosan gel and their effects on microspheres preparation and drug release. Carbohydr Polym. 2000;41:389–396. doi: 10.1016/S0144-8617(99)00104-6. - DOI
-
- Jia LN, Zhang X, Xu HY, Hua F, Hu XG, Xie Q, Wang W, Jia J. Development of a doxycycline hydrochloride-loaded electrospun nanofibrous membrane for GTR/GBR applications. J Nanomater. 2016;2016:1–10. doi: 10.1155/2016/6507459. - DOI
-
- Chandrasekaran AR, Jia CY, Theng CS, Muniandy T, Muralidharan S, Dhanaraj SA. In vitro studies and evaluation of metformin marketed tablets-Malaysia. J Appl Pharm Sci. 2011;1:214–217.
-
- Maganti N, Venkat Surya PKC, Thein-Han WW, Pesacreta TC, Misra RDK. Structure-process-property relationship of biomimetic chitosan-based nanocomposite scaffolds for tissue engineering: biological, physico-chemical, and mechanical functions. Adv Eng Mater. 2011;13:B108–B122. doi: 10.1002/adem.201080094. - DOI
-
- Xianmiao C, Yubao L, Yi Z, Li Z, Jidong L, Huanan W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng C. 2009;29:29–35. doi: 10.1016/j.msec.2008.05.008. - DOI
-
- Kumar TM. Spectroscopic characterization of chloramphenicol and tetracycline: an impact of biofield treatment. Pharm Anal Acta. 2015;6:1–5. doi: 10.4172/2153-2435.1000395. - DOI
-
- Junejo Y, Safdar M. Highly effective heterogeneous doxycycline stabilized silver nanocatalyst for the degradation of ibuprofen and paracetamol drugs. Arab J Chem. 2019;12:2823–2832. doi: 10.1016/j.arabjc.2015.06.014. - DOI
-
- Bin QSS, Zafar MS, Niazi FH, Alshahwan M, HA KS, Daood U. Functionally graded biomimetic biomaterials in dentistry: an evidence-based update. J Biomater Sci Polym Ed. 2020:1–20. 10.1080/09205063.2020.1744289.
-
- Bottino MC, Arthur RA, Waeiss RA, Kamocki K, Gregson KS, Gregory RL. Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig. 2014;18:2151–2158. doi: 10.1007/s00784-014-1201-x. - DOI - PMC - PubMed
-
- Jana S, Florczyk SJ, Leung M, Zhang M. High-strength pristine porous chitosan scaffolds for tissue engineering. J Mater Chem. 2012;22:6291–6299. doi: 10.1039/c2jm16676c. - DOI
-
- Barbosa MA, Pêgo AP, Amaral IF. 2.213 - Chitosan. In: Editor-in-Chief: Paul D, editor. Compr. Biomater., Oxford: Elsevier; 2011, p. 221–37. 10.1016/B978-0-08-055294-1.00072-6.
-
- Yuan NY, Lin YA, Ho MH, Wang DM, Lai JY, Hsieh HJ. Effects of the cooling mode on the structure and strength of porous scaffolds made of chitosan, alginate, and carboxymethyl cellulose by the freeze-gelation method. Carbohydr Polym. 2009;78:349–356. doi: 10.1016/j.carbpol.2009.04.021. - DOI
-
- Roberts G, Taylor K. Chitosan gels. III: the formation of gels by reaction of chitosan with glutaraldehyde. Die Makromol Chemie. 1989;190:951–960. doi: 10.1002/macp.1989.021900504. - DOI
-
- Mirzaei BE, Ramazani A, Shafiee M, Danaei M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int J Polym Mater Polym Biomater. 2013;62:605–611. doi: 10.1080/00914037.2013.769165. - DOI