Unhealthy Phenotype as Indicated by Salivary Biomarkers: Glucose, Insulin, VEGF-A, and IL-12p70 in Obese Kuwaiti Adolescents
Affiliations
Affiliations
- Department of Applied Oral Sciences, The Forsyth Institute, Cambridge, MA 02142, USA.
- Department of Nutrition, Dasman Diabetes Institute, 15462 Dasman, Kuwait.
- Genome Center, The Dasman Diabetes Institute, 15462 Dasman, Kuwait.
- Ministry of Health, 13001 Safat, Kuwait.
- Faculty of Dentistry, Kuwait University, 13060 Safat, Kuwait.
- Dasman Diabetes Institute, 15462 Dasman, Kuwait.
- Division of Cardiology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA.
Abstract
Objective: Here, we investigated the relationships between obesity and the salivary concentrations of insulin, glucose, and 20 metabolic biomarkers in Kuwaiti adolescents. Previously, we have shown that certain salivary metabolic markers can act as surrogates for blood concentrations.
Methods: Salivary samples of whole saliva were collected from 8,317 adolescents. Salivary glucose concentration was measured by a high-sensitivity glucose oxidase method implemented on a robotic chemical analyzer. The concentration of salivary insulin and 20 other metabolic biomarkers was assayed in 744 randomly selected saliva samples by multiplexed bead-based immunoassay.
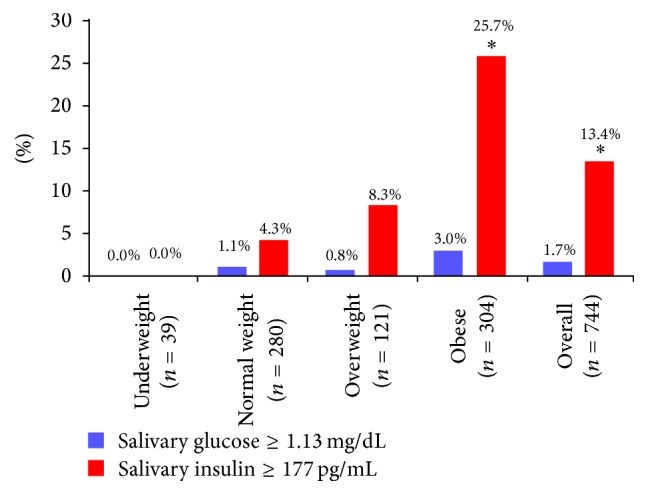
Results: Obesity was seen in 26.5% of the adolescents. Salivary insulin predicting hyperinsulinemia occurred in 4.3% of normal-weight adolescents, 8.3% of overweight adolescents, and 25.7% of obese adolescents (p < 0.0001). Salivary glucose predicting hyperglycemia was found in only 3% of obese children and was not predictive (p = 0.89). Elevated salivary glucose and insulin occurring together was associated with elevated vascular endothelial growth factor and reduced salivary interleukin-12.
Conclusion: Considering the surrogate nature of salivary insulin and glucose, this study suggests that elevated insulin may be a dominant sign of metabolic disease in adolescent populations. It also appears that a proangiogenic environment may accompany elevated glucose in obese adolescents.
Figures
Similar articles
Salivary Biomarkers as Predictors of Obesity and Intermediate Hyperglycemia in Adolescents.
Alqaderi H, Hegazi F, Al-Mulla F, Chiu CJ, Kantarci A, Al-Ozairi E, Abu-Farha M, Bin-Hasan S, Alsumait A, Abubaker J, Devarajan S, Goodson JM, Hasturk H, Tavares M.Front Public Health. 2022 Jun 10;10:800373. doi: 10.3389/fpubh.2022.800373. eCollection 2022.PMID: 35757631 Free PMC article.
Pîrsean C, Neguț C, Stefan-van Staden RI, Dinu-Pirvu CE, Armean P, Udeanu DI.PLoS One. 2019 Jan 3;14(1):e0210288. doi: 10.1371/journal.pone.0210288. eCollection 2019.PMID: 30605486 Free PMC article.
Shi P, Goodson JM.J Obes. 2019 May 19;2019:9570218. doi: 10.1155/2019/9570218. eCollection 2019.PMID: 31236292 Free PMC article.
Salivary Biomarkers in Pediatric Metabolic Disease Research.
Hartman ML, Goodson JM, Barake R, Alsmadi O, Al-Mutawa S, Ariga J, Soparkar P, Behbehani J, Behbehani K.Pediatr Endocrinol Rev. 2016 Mar;13(3):602-11.PMID: 27116847 Review.
Sleep efficiency as a determinant of insulin sensitivity in overweight and obese adolescents.
Dorenbos E, Rijks JM, Adam TC, Westerterp-Plantenga MS, Vreugdenhil AC.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:90-8. doi: 10.1111/dom.12515.PMID: 26332973 Review.
Cited by
An official website of the United States government
Here's how you know
Access keysNCBI HomepageMyNCBI HomepageMain ContentMain Navigation
Search:0 results are available, use up and down arrow keys to navigate.Search
SaveEmail
Send to
Display options
Abstract PubMed PMID
full text links
actions
Cite
Collections
share
page navigation
- Title & authors
- Abstract
- Figures
- Similar articles
- Cited by
- References
- Publication types
- MeSH terms
- Substances
- Related information
- LinkOut - more resources
Title & authors Abstract Figures Similar articles Cited by References Publication types MeSH terms Substances Related information LinkOut - more resources
J Obes
. 2016;2016:6860240.
doi: 10.1155/2016/6860240. Epub 2016 Mar 16.
Unhealthy Phenotype as Indicated by Salivary Biomarkers: Glucose, Insulin, VEGF-A, and IL-12p70 in Obese Kuwaiti Adolescents
Mor-Li Hartman 1, J Max Goodson 1, Ping Shi 1, Jorel Vargas 1, Tina Yaskell 1, Danielle Stephens 1, Maryann Cugini 1, Hatice Hasturk 1, Roula Barake 2, Osama Alsmadi 3, Sabiha Al-Mutawa 4, Jitendra Ariga 4, Pramod Soparkar 1, Jawad Behbehani 5, Kazem Behbehani 6, Francine Welty 7
Affiliations expand
- PMID: 27069678
- PMCID: PMC4812454
- DOI: 10.1155/2016/6860240
Free PMC article
Abstract
Objective: Here, we investigated the relationships between obesity and the salivary concentrations of insulin, glucose, and 20 metabolic biomarkers in Kuwaiti adolescents. Previously, we have shown that certain salivary metabolic markers can act as surrogates for blood concentrations.
Methods: Salivary samples of whole saliva were collected from 8,317 adolescents. Salivary glucose concentration was measured by a high-sensitivity glucose oxidase method implemented on a robotic chemical analyzer. The concentration of salivary insulin and 20 other metabolic biomarkers was assayed in 744 randomly selected saliva samples by multiplexed bead-based immunoassay.
Results: Obesity was seen in 26.5% of the adolescents. Salivary insulin predicting hyperinsulinemia occurred in 4.3% of normal-weight adolescents, 8.3% of overweight adolescents, and 25.7% of obese adolescents (p < 0.0001). Salivary glucose predicting hyperglycemia was found in only 3% of obese children and was not predictive (p = 0.89). Elevated salivary glucose and insulin occurring together was associated with elevated vascular endothelial growth factor and reduced salivary interleukin-12.
Conclusion: Considering the surrogate nature of salivary insulin and glucose, this study suggests that elevated insulin may be a dominant sign of metabolic disease in adolescent populations. It also appears that a proangiogenic environment may accompany elevated glucose in obese adolescents.
Figures

Figure 1
Semilogarithmic distribution of salivary glucose…

Figure 2
Percentage of the subset of…
Similar articles
Salivary Biomarkers as Predictors of Obesity and Intermediate Hyperglycemia in Adolescents.
Alqaderi H, Hegazi F, Al-Mulla F, Chiu CJ, Kantarci A, Al-Ozairi E, Abu-Farha M, Bin-Hasan S, Alsumait A, Abubaker J, Devarajan S, Goodson JM, Hasturk H, Tavares M.Front Public Health. 2022 Jun 10;10:800373. doi: 10.3389/fpubh.2022.800373. eCollection 2022.PMID: 35757631 Free PMC article.
Pîrsean C, Neguț C, Stefan-van Staden RI, Dinu-Pirvu CE, Armean P, Udeanu DI.PLoS One. 2019 Jan 3;14(1):e0210288. doi: 10.1371/journal.pone.0210288. eCollection 2019.PMID: 30605486 Free PMC article.
Shi P, Goodson JM.J Obes. 2019 May 19;2019:9570218. doi: 10.1155/2019/9570218. eCollection 2019.PMID: 31236292 Free PMC article.
Salivary Biomarkers in Pediatric Metabolic Disease Research.
Hartman ML, Goodson JM, Barake R, Alsmadi O, Al-Mutawa S, Ariga J, Soparkar P, Behbehani J, Behbehani K.Pediatr Endocrinol Rev. 2016 Mar;13(3):602-11.PMID: 27116847 Review.
Sleep efficiency as a determinant of insulin sensitivity in overweight and obese adolescents.
Dorenbos E, Rijks JM, Adam TC, Westerterp-Plantenga MS, Vreugdenhil AC.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:90-8. doi: 10.1111/dom.12515.PMID: 26332973 Review.
See all similar articles
Cited by
Salivary phosphate as a biomarker for human diseases.
Razzaque MS.FASEB Bioadv. 2022 Jan 3;4(2):102-108. doi: 10.1096/fba.2021-00104. eCollection 2022 Feb.PMID: 35141474 Free PMC article.
Salivary Redox Biomarkers in Insulin Resistance: Preclinical Studies in an Animal Model.
Maciejczyk M, Pawlukianiec C, Żendzian-Piotrowska M, Ładny JR, Zalewska A.Oxid Med Cell Longev. 2021 Sep 9;2021:3734252. doi: 10.1155/2021/3734252. eCollection 2021.PMID: 34557264 Free PMC article.
Caveolin-1 Variant Is Associated With the Metabolic Syndrome in Kuwaiti Children.
Nizam R, Al-Ozairi E, Goodson JM, Melhem M, Davidsson L, Alkhandari H, Al Madhoun A, Shamsah S, Qaddoumi M, Alghanim G, Alhasawi N, Abu-Farha M, Abubaker J, Shi P, Hartman ML, Tavares M, Bitar M, Ali H, Arefanian H, Devarajan S, Al-Refaei F, Alsmadi O, Tuomilehto J, Al-Mulla F.Front Genet. 2018 Dec 21;9:689. doi: 10.3389/fgene.2018.00689. eCollection 2018.PMID: 30622557 Free PMC article.
Guo Y, Guo LN, Zhu JF, Tang CY, Feng YZ, Zhou HD.Int J Endocrinol. 2017;2017:1087017. doi: 10.1155/2017/1087017. Epub 2017 Oct 4.PMID: 29109737 Free PMC article.
Saliva diagnostics - Current views and directions.
Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F, Wong DT.Exp Biol Med (Maywood). 2017 Mar;242(5):459-472. doi: 10.1177/1535370216681550. Epub 2016 Dec 8.PMID: 27903834 Free PMC article. Review.
KMEL References
References
-
- Goodson J. M., Welty F. K. Using salivary biomarkers to identify children at risk of Type 2 diabetes. Diabetes Management. 2014;4(6):463–465. doi: 10.2217/dmt.14.34. - DOI
-
- International Diabetes Federation. IDF Diabetes Atlas. 5th. Brussels, Belgium: International Diabetes Federation; 2011.
-
- Banerjee R. K., Datta A. G. Salivary peroxidases. Molecular and Cellular Biochemistry. 1986;70(1):21–29. - PubMed
-
- Williams C. L., Hayman L. L., Daniels S. R., et al. Cardiovascular health in childhood: a statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106(1):143–160. doi: 10.1161/01.cir.0000019555.61092.9e. - DOI - PubMed
-
- Jessup A., Harrell J. S. The metabolic syndrome: look for it in children and adolescents, too! Clinical Diabetes. 2005;23(1):26–32. doi: 10.2337/diaclin.23.1.26. - DOI
-
- Byrne M. L., O'Brien-Simpson N. M., Reynolds E. C., et al. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behavior, and Immunity. 2013;34:164–175. doi: 10.1016/j.bbi.2013.08.010. - DOI - PubMed
![Figure 1 Semilogarithmic distribution of salivary glucose concentration values. Approximate values of the concentration of plasma glucose were computed from the equation [plasma = 13.5 × saliva + 84.8] [10]. The salivary glucose concentration of 1.13 mg/dL was computed from the same equation and is approximately equivalent to a hyperglycemic plasma value of 100 mg/dL.](/Images/Figures/12_2242.jpg)