Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans
Affiliations
Affiliations
- Department of Restorative Sciences, Faculty of Dentistry, Kuwait University, Kuwait City 13060, Kuwait.
- Department of Bioclinical Sciences, Faculty of Dentistry, Kuwait University, Kuwait City 13060, Kuwait.
- Dasman Diabetes Institute, Dasman 15462, Kuwait.
Abstract
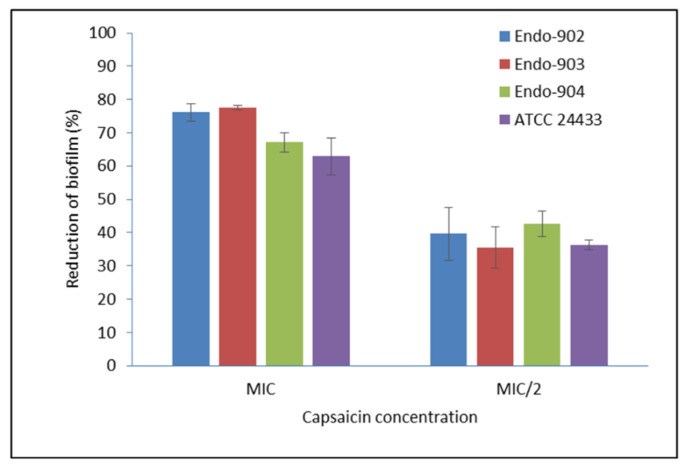
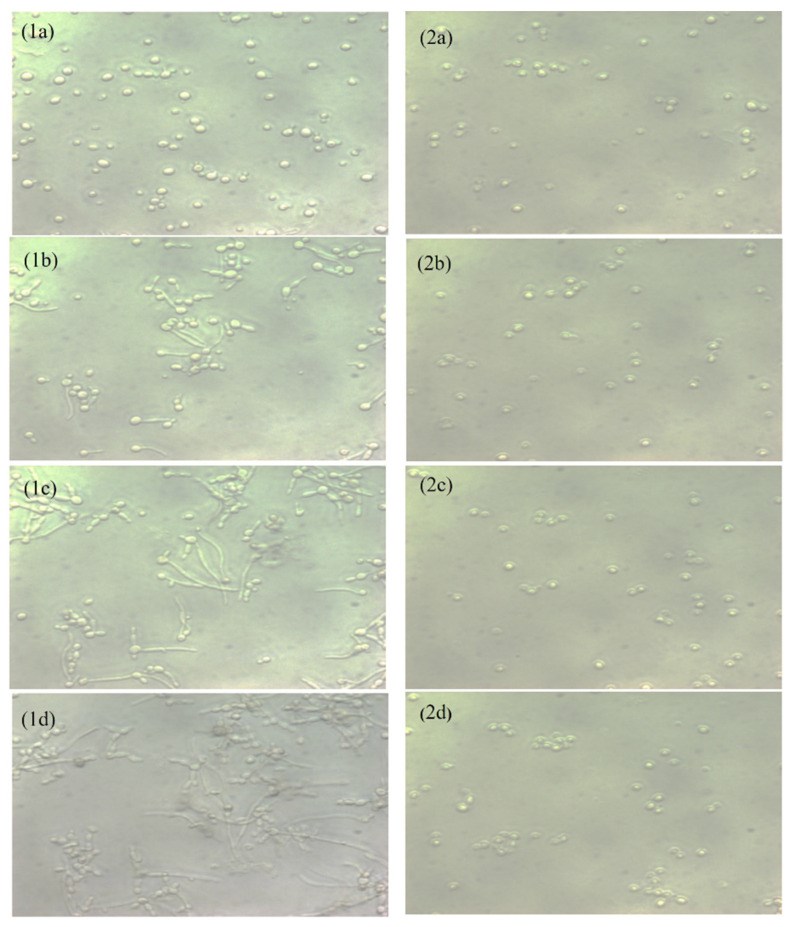
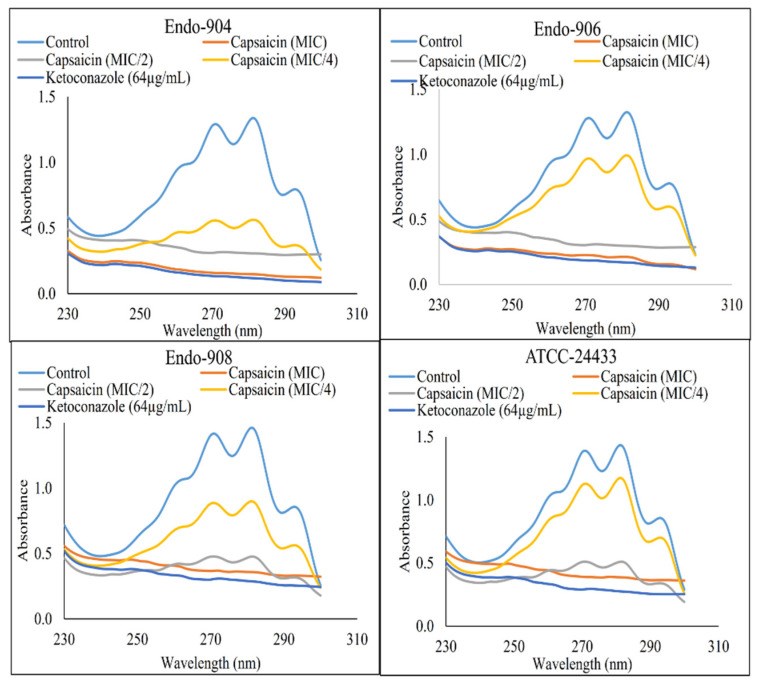
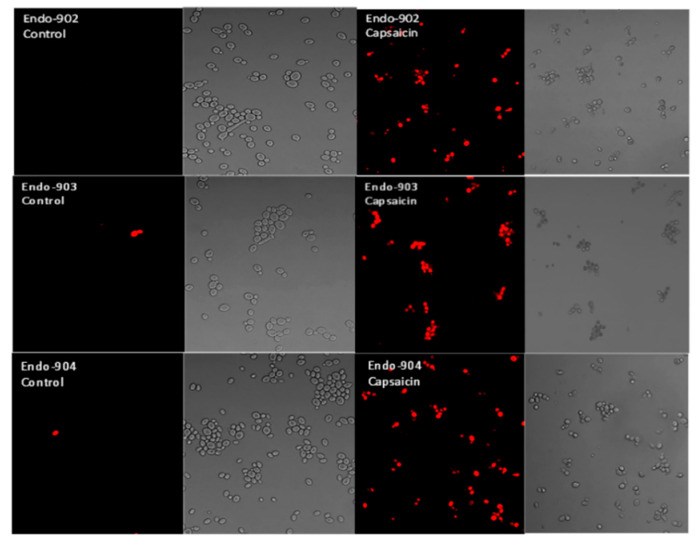
Oral candidiasis is an infection of the oral cavity commonly caused by Candida albicans. Endodontic treatment failure has also been found to be persistent from C. albicans in the root canal system. Despite the availability of antifungal drugs, the management of Candida oral infection is difficult as it exhibits resistance to a different class of antifungal drugs. Therefore, it is necessary to discover new antifungal compounds to cure fungal infections. This study aimed to examine the antifungal susceptibility of Capsaicin, an active compound of chili pepper. The susceptibility of Capsaicin and Fluconazole was tested against the Candida species by the CLSI (M27-A3) method. The effect of Capsaicin on the fungal cell wall was examined by the ergosterol inhibitory assay and observed by the scanning electron micrograph. The MIC range of Capsaicin against Candida isolates from oral (n = 30), endodontic (n = 8), and ATCC strains (n = 2) was 12.5−50 µg/mL. The MIC range of Fluconazole (128- 4 µg/mL) significantly decreased (2- to 4-fold) after the combination with Capsaicin (MIC/4) (p < 0.05). Capsaicin (at MIC) significantly reduced the mature biofilm of C. albicans by 70 to 89% (p < 0.01). The ergosterol content of the cell wall decreased significantly with the increase in the Capsaicin dose (p < 0.01). Capsaicin showed high sensitivity against the hyphae formation and demonstrated a more than 71% reduction in mature biofilm. A fluorescence microscopy revealed the membrane disruption of Capsaicin-treated C. albicans cells, whereas a micrograph of electron microscopy showed the distorted cells’ shape, ruptured cell walls, and shrinkage of cells after the release of intracellular content. The results conclude that Capsaicin had a potential antifungal activity that inhibits the ergosterol biosynthesis in the cell wall, and therefore, the cells’ structure and integrity were disrupted. More importantly, Capsaicin synergistically enhanced the Fluconazole antifungal activity, and the synergistic effect might be helpful in the prevention of Fluconazole resistance development and reduced drug-dosing.
Keywords: Capsaicin; anticandidal; chili pepper; ergosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
Behbehani JM, Irshad M, Shreaz S, Karched M.J Mycol Med. 2019 Jun;29(2):158-167. doi: 10.1016/j.mycmed.2019.01.011. Epub 2019 Feb 20.PMID: 30797684
Esfahani A, Omran AN, Salehi Z, Shams-Ghahfarokhi M, Ghane M, Eybpoosh S, Razzaghi-Abyaneh M.Microb Pathog. 2022 Sep;170:105696. doi: 10.1016/j.micpath.2022.105696. Epub 2022 Jul 31.PMID: 35921954 Review.
Al-Madboly LA, Abd El-Salam MA, Bastos JK, El-Shorbagy SH, El-Morsi RM.Microbiol Spectr. 2022 Oct 26;10(5):e0272421. doi: 10.1128/spectrum.02724-21. Epub 2022 Aug 16.PMID: 35972130 Free PMC article.
Arthington-Skaggs BA, Warnock DW, Morrison CJ.Antimicrob Agents Chemother. 2000 Aug;44(8):2081-5. doi: 10.1128/AAC.44.8.2081-2085.2000.PMID: 10898679 Free PMC article.
Candida and candidaemia. Susceptibility and epidemiology.
Arendrup MC.Dan Med J. 2013 Nov;60(11):B4698.PMID: 24192246 Review.
KMEL References
References
-
- Shah B., Harshe D.G., Shah H., Shetty N., Shenoy A., Ramakrishnan A., Cholera R., Kale S. Perceived hassles and uplifts and their impact on perceived cognitive performance during pregnancy: A pilot study. Endodontology. 2016;28:109–113.
-
- Wisplinghoff H., Ebbers J., Geurtz L., Stefanik D., Major Y., Edmond M.B., Wenzel R.P., Seifert H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents. 2014;43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005. - DOI - PubMed
-
- Jensen R.H., Astvad K.M.T., Silva L.V., Sanglard D., Jørgensen R., Nielsen K.F., Mathiasen E.G., Doroudian G., Perlin D.S., Arendrup M.C. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J. Antimicrob. Chemother. 2015;70:2551–2555. doi: 10.1093/jac/dkv140. - DOI - PMC - PubMed
-
- Soumya S., Nair B.R. Antifungal efficacy of Capsicum frutescens L. extracts against some prevalent fungal strains associated with groundnut storage. J. Agric. Technol. 2012;8:739–750.
-
- Dorantes L., Colmenero R., Hernández H., Mota L., Jaramillo M.E., Fernandez E., Solano C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000;57:125–128. doi: 10.1016/S0168-1605(00)00216-6. - DOI
-
- Menezes R.D.P., Bessa M.A.D.S., Siqueira C.D.P., Teixeira S.C., Ferro E.A.V., Martins M.M., Cunha L.C.S., Martins C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics. 2022;11:1154. doi: 10.3390/antibiotics11091154. - DOI - PMC - PubMed
-
- Buitimea-Cantúa G.V., Velez-Haro J.M., Buitimea-Cantúa N.E., Molina-Torres J., Rosas-Burgos E.C. GC-EIMS analysis, antifungal and anti-aflatoxigenic activity of Capsicum chinense and Aspergillus parasiticus fruits and their bioactive compounds capsaicin and piperine upon Aspergillus parasiticus. Nat. Prod. Res. 2020;34:1452–1455. doi: 10.1080/14786419.2018.1514395. - DOI - PubMed
-
- Hayman M., Kam P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care. 2008;19:338–343. doi: 10.1016/j.cacc.2008.07.003. - DOI
-
- Zhou Y., Guan X., Zhu W., Liu Z., Wang X., Yu H., Wang H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:211–219. doi: 10.1007/s10096-013-1947-0. - DOI - PubMed
-
- Samaranayake L.P., MacFarlane T.W., Lamey P.J., Ferguson M.M. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J. Oral Pathol. 1986;15:386–388. doi: 10.1111/j.1600-0714.1986.tb00646.x. - DOI - PubMed
-
- Wayne P. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008.
-
- Breivik O.N., Owades J.L. Yeast Analysis, Spectrophotometric Semimicrodetermination of Ergosterol in Yeast. J. Agric. Food Chem. 1957;5:360–363. doi: 10.1021/jf60075a005. - DOI