Recombinant growth hormone therapy in children with short stature in Kuwait: a cross-sectional study of use and treatment outcomes
Affiliations
Affiliations
- Department of Pediatrics, Faculty of Medicine, Kuwait University, PO Box 24923, Safat, 13110, Kuwait. d.alabdulrazzaq@hsc.edu.kw.
- Department of Community Medicine, Faculty of Medicine, Kuwait University, PO Box 24923, Safat, 13110, Kuwait. altaiar@hsc.edu.kw.
- Department of Pediatrics, Mubarak Al-Kabeer Hospital, Ministry of Health, Safat, Kuwait. eternity2alex@yahoo.com.
- Department of Pediatrics, Mubarak Al-Kabeer Hospital, Ministry of Health, Safat, Kuwait. ewdln@icloud.com.
Abstract
Background: Recombinant Growth hormone (rGH) therapy is approved in many countries for treatment of short stature in a number of childhood diagnoses. Despite the increasing body of international literature on rGH use, there is paucity of data on rGH use in Kuwait and the broader Middle-East which share unique ethnic and socio-cultural backgrounds. This study aimed to describe the pattern of use and treatment outcomes of rGH therapy in Kuwait.
Methods: This is a cross-sectional retrospective review of children treated with rGH in the Department of Pediatrics, in a major hospital in Kuwait between December 2013 and December 2014. Data were extracted using standard data extraction form and the response to rGH therapy was defined as a gain of ≥ 0.3 standard deviation score (SDS) of height per year.
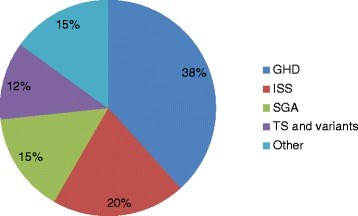
Results: A total of 60 children were treated with rGH in the center. Their Median (Interquartile) age at rGH initiation was 9.0 (6.2, 10.7) years. The most common indications for rGH therapy were Growth Hormone Deficiency (GHD) 23 (38.3 %), Idiopathic Short Stature (ISS) 12 (20.0 %) and Small for Gestational Age (SGA) 9 (15.0 %). After excluding patients with TS, no significant differences were found in gender of those who received rGH therapy in all indications combined or in each group (p ≥ 0.40). At 1-year follow-up, children in all groups had median height SDS change of ≥ 0.3 SDS except for children with ISS. Age at rGH initiation was negatively associated with 1-year treatment response, Adjusted odds ratio (AOR) 0.56 (95 % CI: 0.04-1.49); p = 0.011).
Conclusions: GHD is the most common indication of rGH therapy. All indications except for ISS showed significant 1-year treatment response to therapy. Treatment outcomes in patients with ISS should be further investigated in Kuwait. Younger age at initiation of rGH therapy was independently associated with significant response to therapy suggesting the importance of identifying children with short stature and prompt initiation of rGH therapy.
Figures
Similar articles
Colmenares A, González L, Gunczler P, Lanes R.J Pediatr Endocrinol Metab. 2012;25(7-8):651-7. doi: 10.1515/jpem-2012-0182.PMID: 23155689
Hughes IP, Harris M, Choong CS, Ambler G, Cutfield W, Hofman P, Cowell CT, Werther G, Cotterill A, Davies PS; Australasian Paediatric Endocrine Group (APEG).Clin Endocrinol (Oxf). 2012 Jul;77(1):62-71. doi: 10.1111/j.1365-2265.2011.04230.x.PMID: 21950731
Al Shaikh A, Daftardar H, Alghamdi AA, Jamjoom M, Awidah S, Ahmed ME, Soliman AT.Acta Biomed. 2020 Mar 19;91(1):29-40. doi: 10.23750/abm.v91i1.9182.PMID: 32191651 Free PMC article.
Ranke MB, Lindberg A.Horm Res Paediatr. 2011;75(6):423-32. doi: 10.1159/000324117. Epub 2011 Feb 25.PMID: 21358173 Review.
Growth hormone treatment for short stature in children born small for gestational age.
Jung H, Rosilio M, Blum WF, Drop SL.Adv Ther. 2008 Oct;25(10):951-78. doi: 10.1007/s12325-008-0101-3.PMID: 18836868 Review.
Cited by
Kaplan W, Al Amiri E, Attia N, Al Basiri I, Romany I, Al Shehri E, Al Twaim A, Al Yaarubi S, Deeb A.Front Pediatr. 2022 Nov 25;10:988614. doi: 10.3389/fped.2022.988614. eCollection 2022.PMID: 36507126 Free PMC article.
Height outcomes in Korean children with idiopathic short stature receiving growth hormone treatment.
Chae HW, Hwang IT, Lee JE, So CH, Rhie YJ, Lim JS, Kwon EB, Yi KH, Kim EY, Jo CK, Shim KS, Gil HY, Seong MJ, Nam CM, Moon JS, Hwang JS.Front Endocrinol (Lausanne). 2022 Sep 7;13:925102. doi: 10.3389/fendo.2022.925102. eCollection 2022.PMID: 36157444 Free PMC article.
Kochar IS, Ramachandran S, Sethi A.Indian J Endocrinol Metab. 2021 Jan-Feb;25(1):54-58. doi: 10.4103/ijem.IJEM_739_20. Epub 2021 Jul 21.PMID: 34386395 Free PMC article.
Li S, Wang X, Zhao Y, Ji W, Mao J, Nie M, Wu X.Endocrine. 2020 Sep;69(3):615-624. doi: 10.1007/s12020-020-02375-5. Epub 2020 Jun 12.PMID: 32533506
Alzahrani AK, Algethami AK, Barnawi G, Meftah IA, Alshanqiti A Sr, Al-Hashmi H, Khan MA, Felimban N.Cureus. 2020 Mar 18;12(3):e7319. doi: 10.7759/cureus.7319.PMID: 32313760 Free PMC article.
KMEL References
References
-
- Lee PA, Sävendahl L, Oliver I, Tauber M, Blankenstein O, Ross J, et al. Comparison of response to 2-years' growth hormone treatment in children with isolated growth hormone deficiency, born small for gestational age, idiopathic short stature, or multiple pituitary hormone deficiency: combined results from two large observational studies. Int J Pediatr Endocrinol. 2012;2012(1):22. doi: 10.1186/1687-9856-2012-22. - DOI - PMC - PubMed
-
- Carel JC, Ecosse E, Nicolino M, Tauber M, Leger J, Cabrol S, et al. Adult height after long term treatment with recombinant growth hormone for idiopathic isolated growth hormone deficiency: observational follow up study of the French population based registry. BMJ. 2002;325(7355):70. doi: 10.1136/bmj.325.7355.70. - DOI - PMC - PubMed
-
- Bryant J, Djurhuus CB, Christensen T, Jøns K, Hokken-Koelega A. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007;3 - PubMed
-
- Public Authority for Civil Information (PACI), Population Statistical Reports. 2014 [cited 2015 July 12th]; Available from: https://www.paci.gov.kw/Home.aspx.
-
- Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210–7. doi: 10.1210/jc.2008-0509. - DOI - PubMed
-
- Lee PA, Chernausek SD, Hokken-Koelega AC. Czernichow P; International Small for Gestational Age Advisory Board. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111(6 Pt 1):1253–61. doi: 10.1542/peds.111.6.1253. - DOI - PubMed
-
- Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92(3):804–10. doi: 10.1210/jc.2006-2017. - DOI - PubMed
-
- Group, W.H.O.M.G.R.S. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. - PubMed
-
- Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–25. doi: 10.1210/jc.2011-1995. - DOI - PubMed
-
- Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. 2006;91(6):2047–54. doi: 10.1210/jc.2005-2284. - DOI - PubMed
-
- Ranke MB, Lindberg A, Price DA, Darendeliler F, Albertsson-Wikland K, Wilton P, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Horm Res. 2007;68(2):53–62. doi: 10.1159/000098707. - DOI - PubMed
-
- Growth Hormone Research S. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3. - PubMed
-
- Rose SR, Shulman DI, Larsson P, Wakley LR, Wills S, Bakker B. Gender does not influence prepubertal growth velocity during standard growth hormone therapy--analysis of United States KIGS data. J Pediatr Endocrinol Metab. 2005;18(11):1045–51. - PubMed